Organisation of sensitisation of hind limb withdrawal reflexes from acute noxious stimuli in the rabbit
- PMID: 12509493
- PMCID: PMC2342464
- DOI: 10.1113/jphysiol.2002.025023
Organisation of sensitisation of hind limb withdrawal reflexes from acute noxious stimuli in the rabbit
Abstract
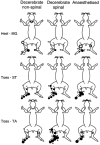
Spatial aspects of central sensitisation were investigated by studying the effects on three hind limb withdrawal reflexes of an acute noxious stimulus (20 % mustard oil) applied to a number of locations around the body in decerebrate and in anaesthetised rabbits. Reflex responses to electrical stimulation of the toes were recorded from the ankle flexor tibialis anterior (TA) and the knee flexor semitendinosus (ST), whereas responses to stimulation of the heel were recorded from the ankle extensor medial gastrocnemius (MG). In non-spinalised, decerebrated, pentobarbitone-sedated preparations, flexor reflexes were facilitated significantly from sites on the plantar surface of the ipsilateral foot but were either inhibited or unaffected by stimulation of sites away from this location. The heel-MG reflex was facilitated from the ipsilateral heel and was inhibited from a number of ipsilateral, contralateral and off-limb sites. In decerebrated, spinalised, pentobarbitone-sedated animals, mustard oil applied to any site on the ipsilateral hind limb enhanced both flexor reflexes, whereas the MG reflex was enhanced only after stimulation at the ipsilateral heel and was inhibited after stimulation of the toe tips or TA muscle. Mustard oil on the contralateral limb had no effect on any reflex. In rabbits anaesthetised with pentobarbitone and prepared with minimal surgical interference, the sensitisation fields for the heel-MG and toes-TA reflexes were very similar to those in non-spinal decerebrates whereas that for toes-ST was more like the pattern observed in spinalised animals. In no preparation was sensitisation or inhibition of reflexes related to the degree of motoneurone activity generated in direct response to the sensitising stimulus. This study provides for the first time a complete description of the sensitisation fields for reflexes to individual muscles. Descending controls had a marked effect on the area from which sensitisation of flexor reflexes could be obtained, as the sensitisation fields for the flexor reflexes evoked from the toes were larger in spinalised compared to decerebrated, non-spinalised animals. The intermediate sizes of sensitisation fields in anaesthetised animals suggests that the area of these fields can be dynamically controlled from the brain. On the other hand, the sensitisation field for the heel-MG reflex varied little between preparations and appears to be a function of spinal neurones.
Figures





Similar articles
-
Involvement of spinal α2 -adrenoceptors in prolonged modulation of hind limb withdrawal reflexes following acute noxious stimulation in the anaesthetized rabbit.Eur J Neurosci. 2016 Mar;43(6):834-45. doi: 10.1111/ejn.13185. Epub 2016 Feb 28. Eur J Neurosci. 2016. PMID: 26804327 Free PMC article.
-
Differences in opioidergic inhibition of spinal reflexes and Fos expression evoked by mechanical and chemical noxious stimuli in the decerebrated rabbit.Neuroscience. 1999 Apr;90(1):177-89. doi: 10.1016/s0306-4522(98)00426-6. Neuroscience. 1999. PMID: 10188944
-
Glutamate and tachykinin receptors in central sensitization of withdrawal reflexes in the decerebrated rabbit.Exp Physiol. 2004 Mar;89(2):187-98. doi: 10.1113/expphysiol.2003.002646. Exp Physiol. 2004. PMID: 15123548
-
Contributions to the understanding of gait control.Dan Med J. 2014 Apr;61(4):B4823. Dan Med J. 2014. PMID: 24814597 Review.
-
The organization of motor responses to noxious stimuli.Brain Res Brain Res Rev. 2004 Oct;46(2):163-72. doi: 10.1016/j.brainresrev.2004.07.005. Brain Res Brain Res Rev. 2004. PMID: 15464205 Review.
Cited by
-
Activation of peripheral nerve fibers by electrical stimulation in the sole of the foot.BMC Neurosci. 2013 Oct 8;14:116. doi: 10.1186/1471-2202-14-116. BMC Neurosci. 2013. PMID: 24103294 Free PMC article.
-
Spinal spatial integration of nociception and its functional role assessed via the nociceptive withdrawal reflex and psychophysical measures in healthy humans.Physiol Rep. 2020 Nov;8(22):e14648. doi: 10.14814/phy2.14648. Physiol Rep. 2020. PMID: 33217191 Free PMC article.
-
Involvement of spinal α2 -adrenoceptors in prolonged modulation of hind limb withdrawal reflexes following acute noxious stimulation in the anaesthetized rabbit.Eur J Neurosci. 2016 Mar;43(6):834-45. doi: 10.1111/ejn.13185. Epub 2016 Feb 28. Eur J Neurosci. 2016. PMID: 26804327 Free PMC article.
-
Alfaxalone Anaesthesia Facilitates Electrophysiological Recordings of Nociceptive Withdrawal Reflexes in Dogs (Canis familiaris).PLoS One. 2016 Jul 19;11(7):e0158990. doi: 10.1371/journal.pone.0158990. eCollection 2016. PLoS One. 2016. PMID: 27433936 Free PMC article.
-
Ice water immersion does not activate diffuse noxious inhibitory controls of spinal reflexes in sedated or anaesthetised dogs (Canis familiaris): a pilot study.Front Pain Res (Lausanne). 2025 Mar 10;6:1505064. doi: 10.3389/fpain.2025.1505064. eCollection 2025. Front Pain Res (Lausanne). 2025. PMID: 40129491 Free PMC article.
References
-
- Andersen OK, Eichenberger U, Arendt-Nielsen L. Reflex receptive field size variation following intramuscular capsaicin injection in humans. Proc Xth World Congress on Pain. 2002;517
-
- Andersen OK, Sonnenborg FA, Arendt-Nielsen T. Modular organization of human leg withdrawal reflexes elicited by electrical stimulation of the foot sole. Muscle & Nerve. 1999;22:1520–1530. - PubMed
-
- Babenko V, Graven-Nielsen T, Svensson P, Drewes AM, Jensen TS, Arendt-Nielsen L. Experimental human muscle pain and muscular hyperalgesia induced by combinations of serotonin and bradykinin. Pain. 1999;82:1–8. - PubMed
-
- Babenko V, Svensson P, Graven-Nielsen T, Drewes AM, Jensen TS, Arendt-Nielsen L. Duration and distribution of experimental muscle hyperalgesia in humans following combined infusions of serotonin and bradykinin. Brain Res. 2000;853:275–281. - PubMed
-
- Bhandari RNB, Ogilvie J, Clarke RW. Differences in opioidergic inhibition of spinal reflexes and Fos expression evoked by mechanical and chemical noxious stimuli in the decerebrate rabbit. Neurosci. 1999;90:177–189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

