Hysteresis drives cell-cycle transitions in Xenopus laevis egg extracts
- PMID: 12509509
- PMCID: PMC298711
- DOI: 10.1073/pnas.0235349100
Hysteresis drives cell-cycle transitions in Xenopus laevis egg extracts
Abstract
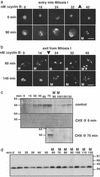
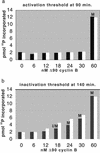
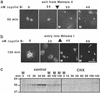
Cells progressing through the cell cycle must commit irreversibly to mitosis without slipping back to interphase before properly segregating their chromosomes. A mathematical model of cell-cycle progression in cell-free egg extracts from frog predicts that irreversible transitions into and out of mitosis are driven by hysteresis in the molecular control system. Hysteresis refers to toggle-like switching behavior in a dynamical system. In the mathematical model, the toggle switch is created by positive feedback in the phosphorylation reactions controlling the activity of Cdc2, a protein kinase bound to its regulatory subunit, cyclin B. To determine whether hysteresis underlies entry into and exit from mitosis in cell-free egg extracts, we tested three predictions of the Novak-Tyson model. (i) The minimal concentration of cyclin B necessary to drive an interphase extract into mitosis is distinctly higher than the minimal concentration necessary to hold a mitotic extract in mitosis, evidence for hysteresis. (ii) Unreplicated DNA elevates the cyclin threshold for Cdc2 activation, indication that checkpoints operate by enlarging the hysteresis loop. (iii) A dramatic "slowing down" in the rate of Cdc2 activation is detected at concentrations of cyclin B marginally above the activation threshold. All three predictions were validated. These observations confirm hysteresis as the driving force for cell-cycle transitions into and out of mitosis.
Figures

Comment in
-
Hysteresis meets the cell cycle.Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):771-2. doi: 10.1073/pnas.0430083100. Epub 2003 Jan 27. Proc Natl Acad Sci U S A. 2003. PMID: 12552095 Free PMC article. Review. No abstract available.
References
-
- Murray A W, Kirschner M W. Nature. 1989;339:275–280. - PubMed
-
- Murray A W. Methods Cell Biol. 1991;36:581–605. - PubMed
-
- Gautier J, Minshull J, Lohka M, Glotzer M, Hunt T, Maller J. Cell. 1990;1990:487–494. - PubMed
-
- Solomon M J, Glotzer M, Lee T H, Phillippe M, Kirschner M W. Cell. 1990;63:1013–1024. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

