Oxidative remodeling of chromoplast carotenoids: identification of the carotenoid dioxygenase CsCCD and CsZCD genes involved in Crocus secondary metabolite biogenesis
- PMID: 12509521
- PMCID: PMC143450
- DOI: 10.1105/tpc.006536
Oxidative remodeling of chromoplast carotenoids: identification of the carotenoid dioxygenase CsCCD and CsZCD genes involved in Crocus secondary metabolite biogenesis
Abstract
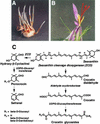
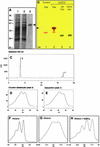
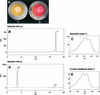
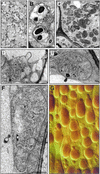
The accumulation of three major carotenoid derivatives-crocetin glycosides, picrocrocin, and safranal-is in large part responsible for the color, bitter taste, and aroma of saffron, which is obtained from the dried styles of Crocus. We have identified and functionally characterized the Crocus zeaxanthin 7,8(7',8')-cleavage dioxygenase gene (CsZCD), which codes for a chromoplast enzyme that initiates the biogenesis of these derivatives. The Crocus carotenoid 9,10(9',10')-cleavage dioxygenase gene (CsCCD) also has been cloned, and the comparison of substrate specificities between these two enzymes has shown that the CsCCD enzyme acts on a broader range of precursors. CsZCD expression is restricted to the style branch tissues and is enhanced under dehydration stress, whereas CsCCD is expressed constitutively in flower and leaf tissues irrespective of dehydration stress. Electron microscopy revealed that the accumulation of saffron metabolites is accompanied by the differentiation of amyloplasts and chromoplasts and by interactions between chromoplasts and the vacuole. Our data suggest that a stepwise sequence exists that involves the oxidative cleavage of zeaxanthin in chromoplasts followed by the sequestration of modified water-soluble derivatives into the central vacuole.
Figures




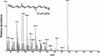


References
-
- Azuma, H., Toyota, M., Asakawa, Y., Takaso, T., and Tobe, H. (2002). Floral scent chemistry of mangrove plants. J. Plant Res. 115, 47–53. - PubMed
-
- Bosser, A., and Belin, J.M. (1994). Synthesis of β-ionone in an aldehyde/xanthine oxidase/β-carotene system involving free radical formation. Biotechnol. Prog. 10, 129–133.
-
- Bouvier, F., Backhaus, R.A., and Camara, B. (1998. a). Induction and control of chromoplast-specific carotenoid genes by oxidative stress. J. Biol. Chem. 273, 30651–30659. - PubMed
-
- Bouvier, F., d'Harlingue, A., Hugueney, P., Marin, E., Marion-Poll, A., and Camara, B. (1996). Xanthophyll biosynthesis: Cloning, expression, functional reconstitution, and regulation of β-cyclohexenyl carotenoid epoxidase from pepper (Capsicum annuum). J. Biol. Chem. 271, 28861–28867. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

