Altered cell cycle distribution, hyperplasia, and inhibited differentiation in Arabidopsis caused by the D-type cyclin CYCD3
- PMID: 12509523
- PMCID: PMC143452
- DOI: 10.1105/tpc.004838
Altered cell cycle distribution, hyperplasia, and inhibited differentiation in Arabidopsis caused by the D-type cyclin CYCD3
Abstract
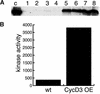
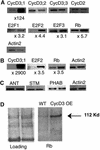
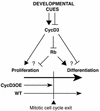
CYCD3;1 expression in Arabidopsis is associated with proliferating tissues such as meristems and developing leaves but not with differentiated tissues. Constitutive overexpression of CYCD3;1 increases CYCD3;1-associated kinase activity and reduces the proportion of cells in the G1-phase of the cell cycle. Moreover, CYCD3;1 overexpression leads to striking alterations in development. Leaf architecture in overexpressing plants is altered radically, with a failure to develop distinct spongy and palisade mesophyll layers. Associated with this, we observe hyperproliferation of leaf cells; in particular, the epidermis consists of large numbers of small, incompletely differentiated polygonal cells. Endoreduplication, a marker for differentiated cells that have exited from the mitotic cell cycle, is inhibited strongly in CYCD3;1-overexpressing plants. Transcript analysis reveals an activation of putative compensatory mechanisms upon CYCD3;1 overexpression or subsequent cell cycle activation. These results demonstrate that cell cycle exit in the G1-phase is required for normal cellular differentiation processes during plant development and suggest a critical role for CYCD3 in the switch from cell proliferation to the final stages of differentiation.
Figures




References
-
- Barton, M.K., and Poethig, R.S. (1993). Formation of the shoot apical meristem in Arabidopsis thaliana: An analysis of development in the wild type and in the shoot meristemless mutant. Development 119, 823–831.
-
- Besnard-Wibaut, C. (1977). Histoautoradiographic analysis of the thermoinductive processes in the shoot apex of Arabidopsis thaliana L. Heynh, vernalised at different stages of development. Plant Cell Physiol. 18, 949–962.
-
- Bowman, J. (1993). Vegetative development. In Arabidopsis: An Atlas of Morphology and Development, J. Bowman, ed (New York: Springer-Verlag), pp.1–89.
-
- Cockcroft, C.E., den Boer, B.G., Healy, J.M., and Murray, J.A. (2000). Cyclin D control of growth rate in plants. Nature 405, 575–579. - PubMed
-
- Colon-Carmona, A., You, R., Haimovitch-Gal, T., and Doerner, P. (1999). Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J. 20, 503–508. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

