The nitrate transporter AtNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in Arabidopsis
- PMID: 12509525
- PMCID: PMC143464
- DOI: 10.1105/tpc.006312
The nitrate transporter AtNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in Arabidopsis
Abstract
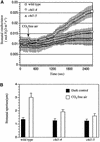
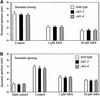
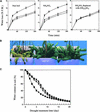
The movement of guard cells in stomatal complexes controls water loss and CO(2) uptake in plants. Examination of the dual-affinity nitrate transporter gene AtNRT1.1 (CHL1) revealed that it is expressed and functions in Arabidopsis guard cells. CHL1 promoter-beta-glucuronidase and CHL1 promoter-green fluorescent protein constructs showed strong expression in guard cells, and immunolocalization experiments with anti-CHL1 antibody confirmed these results. To assess CHL1 function, chl1 mutant plants grown in the presence of nitrate were examined. Compared with wild-type plants, chl1 mutants had reduced stomatal opening and reduced transpiration rates in the light or when deprived of CO(2) in the dark. These effects result in enhanced drought tolerance in chl1 mutants. At the cellular level, chl1 mutants showed reduced nitrate accumulation in guard cells during stomatal opening and failed to show nitrate-induced depolarization of guard cells. In wild-type guard cells, nitrate induced depolarization, and nitrate concentrations increased threefold during stomatal opening. These results identify an anion transporter that functions in stomatal opening and demonstrate that CHL1 supports stomatal function in the presence of nitrate.
Figures




Comment in
-
Can plants rely on nitrate?Trends Plant Sci. 2003 Jul;8(7):314-5; author reply 315-6. doi: 10.1016/S1360-1385(03)00125-0. Trends Plant Sci. 2003. PMID: 12878013 No abstract available.
References
-
- Allaway, W.G. (1973). Accumulation of malate in guard cells of Vicia faba during stomatal opening. Planta 110, 63–70. - PubMed
-
- Assmann, S.M., and Wang, X.-Q. (2001). From milliseconds to millions of years: Guard cells and environmental responses. Curr. Opin. Plant Biol. 4, 421–428. - PubMed
-
- Blatt, M.R. (2000). Cellular signaling and volume control in stomatal movements in plants. Annu. Rev. Cell Dev. Biol. 16, 221–241. - PubMed
-
- Broadley, M.R., Escobar-Gutierrez, A.J., Burns, A., and Burns, I.G. (2001). Nitrogen-limited growth of lettuce is associated with lower stomatal conductance. New Phytol. 152, 97–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

