AtATM is essential for meiosis and the somatic response to DNA damage in plants
- PMID: 12509526
- PMCID: PMC143473
- DOI: 10.1105/tpc.006577
AtATM is essential for meiosis and the somatic response to DNA damage in plants
Abstract
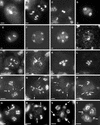
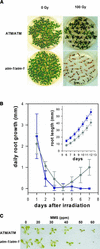
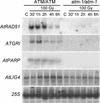
In contrast to yeast or mammalian cells, little is known about the signaling responses to DNA damage in plants. We previously characterized AtATM, an Arabidopsis homolog of the human ATM gene, which is mutated in ataxia telangiectasia, a chromosome instability disorder. The Atm protein is a protein kinase whose activity is induced by DNA damage, particularly DNA double-strand breaks. The phosphorylation targets of Atm include proteins involved in DNA repair, cell cycle control, and apoptosis. Here, we describe the isolation and functional characterization of two Arabidopsis mutants carrying a T-DNA insertion in AtATM. Arabidopsis atm mutants are hypersensitive to gamma-radiation and methylmethane sulfonate but not to UV-B light. In correlation with the radiation sensitivity, atm mutants failed to induce the transcription of genes involved in the repair and/or detection of DNA breaks upon irradiation. In addition, atm mutants are partially sterile, and we show that this effect is attributable to abundant chromosomal fragmentation during meiosis. Interestingly, the transcription of DNA recombination genes during meiosis was not dependent on AtATM, and meiotic recombination occurred at the same rate as in wild-type plants, raising questions about the function of AtAtm during meiosis in plants. Our results demonstrate that AtATM plays a central role in the response to both stress-induced and developmentally programmed DNA damage.
Figures



Comment on
-
ATM to the rescue: repairing DNA damage.Plant Cell. 2003 Jan;15(1):1-3. doi: 10.1105/tpc.150110. Plant Cell. 2003. PMID: 12509517 Free PMC article. No abstract available.
Similar articles
-
ATM to the rescue: repairing DNA damage.Plant Cell. 2003 Jan;15(1):1-3. doi: 10.1105/tpc.150110. Plant Cell. 2003. PMID: 12509517 Free PMC article. No abstract available.
-
NBS1 is involved in DNA repair and plays a synergistic role with ATM in mediating meiotic homologous recombination in plants.Plant J. 2007 Oct;52(1):41-52. doi: 10.1111/j.1365-313X.2007.03220.x. Epub 2007 Aug 2. Plant J. 2007. PMID: 17672843
-
ATM-mediated transcriptional and developmental responses to gamma-rays in Arabidopsis.PLoS One. 2007 May 9;2(5):e430. doi: 10.1371/journal.pone.0000430. PLoS One. 2007. PMID: 17487278 Free PMC article.
-
An insight into the mechanism of DNA damage response in plants- role of SUPPRESSOR OF GAMMA RESPONSE 1: An overview.Mutat Res. 2020 Jan-Apr;819-820:111689. doi: 10.1016/j.mrfmmm.2020.111689. Epub 2020 Jan 23. Mutat Res. 2020. PMID: 32004947 Review.
-
Molecular mechanisms of DNA damage and repair: progress in plants.Crit Rev Biochem Mol Biol. 2001;36(4):337-97. doi: 10.1080/20014091074219. Crit Rev Biochem Mol Biol. 2001. PMID: 11563486 Review.
Cited by
-
Zeocin-induced DNA damage response in barley and its dependence on ATR.Sci Rep. 2024 Feb 7;14(1):3119. doi: 10.1038/s41598-024-53264-0. Sci Rep. 2024. PMID: 38326519 Free PMC article.
-
Mechanisms of stress response in the root stem cell niche.J Exp Bot. 2021 Oct 13;72(19):6746-6754. doi: 10.1093/jxb/erab274. J Exp Bot. 2021. PMID: 34111279 Free PMC article. Review.
-
Arabidopsis ATM and ATR kinases prevent propagation of genome damage caused by telomere dysfunction.Plant Cell. 2011 Dec;23(12):4254-65. doi: 10.1105/tpc.111.092387. Epub 2011 Dec 9. Plant Cell. 2011. PMID: 22158468 Free PMC article.
-
DNA Double-Strand Break Repairs and Their Application in Plant DNA Integration.Genes (Basel). 2022 Feb 9;13(2):322. doi: 10.3390/genes13020322. Genes (Basel). 2022. PMID: 35205367 Free PMC article. Review.
-
Suppressor of gamma response 1 (SOG1) encodes a putative transcription factor governing multiple responses to DNA damage.Proc Natl Acad Sci U S A. 2009 Aug 4;106(31):12843-8. doi: 10.1073/pnas.0810304106. Epub 2009 Jun 19. Proc Natl Acad Sci U S A. 2009. PMID: 19549833 Free PMC article.
References
-
- Alexander, M.P. (1969). Differential staining of aborted and nonaborted pollen. Stain Technol. 44, 117–122. - PubMed
-
- Andegeko, Y., Moyal, L., Mitelman, L., Tsarfaty, I., Shiloh, Y., and Rotman, G. (2001). Nuclear retention of ATM at sites of DNA double strand breaks. J. Biol. Chem. 276, 38224–38230. - PubMed
-
- Angelis, K.J., McGuffie, M., Menke, M., and Schubert, I. (2000). Adaptation to alkylation damage in DNA measured by the comet assay. Environ. Mol. Mutagen. 36, 146–150. - PubMed
-
- Banin, S., Moyal, L., Shieh, S., Taya, Y., Anderson, C.W., Chessa, L., Smorodinsky, N.I., Prives, C., Reiss, Y., Shiloh, Y., and Ziv, Y. (1998). Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281, 1674–1677. - PubMed
-
- Barlow, C., Liyanage, M., Moens, P.B., Deng, C.X., Ried, T., and Wynshaw-Boris, A. (1997). Partial rescue of the prophase I defects of Atm-deficient mice by p53 and p21 null alleles. Nat. Genet 17, 462–466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

