Actin-depolymerizing factor mediates Rac/Rop GTPase-regulated pollen tube growth
- PMID: 12509534
- PMCID: PMC143494
- DOI: 10.1105/tpc.007153
Actin-depolymerizing factor mediates Rac/Rop GTPase-regulated pollen tube growth
Abstract
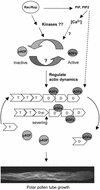
Pollen tube elongation is a rapid tip growth process that is driven by a dynamic actin cytoskeleton. A ubiquitous family of actin binding proteins, actin-depolymerizing factors (ADFs)/cofilins, bind to actin filaments, induce severing, enhance depolymerization from their slow-growing end, and are important for maintaining actin dynamics in vivo. ADFs/cofilins are regulated by multiple mechanisms, among which Rho small GTPase-activated phosphorylation at a terminal region Ser residue plays an important role in regulating their actin binding and depolymerizing activity, affecting actin reorganization. We have shown previously that a tobacco pollen-specific ADF, NtADF1, is important for maintaining normal pollen tube actin cytoskeleton organization and growth. Here, we show that tobacco pollen grains accumulate phosphorylated and nonphosphorylated forms of ADFs, suggesting that phosphorylation could be a regulatory mechanism for their activity. In plants, Rho-related Rac/Rop GTPases have been shown to be important regulators for pollen tube growth. Overexpression of Rac/Rop GTPases converts polar growth into isotropic growth, resulting in pollen tubes with ballooned tips and a disrupted actin cytoskeleton. Using the Rac/Rop GTPase-induced defective pollen tube phenotype as a functional assay, we show that overexpression of NtADF1 suppresses the ability of NtRac1, a tobacco Rac/Rop GTPase, to convert pollen tube tip growth to isotropic growth. This finding suggests that NtADF1 acts in a common pathway with NtRac1 to regulate pollen tube growth. A mutant form of NtADF1 with a nonphosphorylatable Ala substitution at its Ser-6 position [NtADF1(S6A)] shows increased activity, whereas the mutant NtADF1(S6D), which has a phospho-mimicking Asp substitution at the same position, shows reduced ability to counteract the effect of NtRac1. These observations suggest that phosphorylation at Ser-6 of NtADF1 could be important for its integration into the NtRac1 signaling pathway. Moreover, overexpression of NtRac1 diminishes the actin binding activity of green fluorescent protein (GFP)-NtADF1 but has little effect on the association of GFP-NtADF1(S6A) with actin cables in pollen tubes. Together, these observations suggest that NtRac1-activated activity regulates the actin binding and depolymerizing activity of NtADF1, probably via phosphorylation at Ser-6. This notion is further supported by the observation that overexpressing a constitutively active NtRac1 in transformed pollen grains significantly increases the ratio of phosphorylated to nonphosphorylated ADFs. Together, the observations reported here strongly support the idea that NtRac1 modulates NtADF1 activity through phosphorylation at Ser-6 to regulate actin dynamics.
Figures




Similar articles
-
The regulation of actin organization by actin-depolymerizing factor in elongating pollen tubes.Plant Cell. 2002 Sep;14(9):2175-90. doi: 10.1105/tpc.003038. Plant Cell. 2002. PMID: 12215514 Free PMC article.
-
Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes.J Cell Biol. 2001 Mar 5;152(5):1019-32. doi: 10.1083/jcb.152.5.1019. J Cell Biol. 2001. PMID: 11238457 Free PMC article.
-
In vivo Rac/Rop localization as well as interaction with RhoGAP and RhoGDI in tobacco pollen tubes: analysis by low-level expression of fluorescent fusion proteins and bimolecular fluorescence complementation.Plant J. 2015 Oct;84(1):83-98. doi: 10.1111/tpj.12961. Epub 2015 Sep 15. Plant J. 2015. PMID: 26252733
-
ROP GTPase regulation of pollen tube growth through the dynamics of tip-localized F-actin.J Exp Bot. 2003 Jan;54(380):93-101. doi: 10.1093/jxb/erg035. J Exp Bot. 2003. PMID: 12456759 Review.
-
Regulating actin dynamics in neuronal growth cones by ADF/cofilin and rho family GTPases.J Neurobiol. 2000 Aug;44(2):126-44. J Neurobiol. 2000. PMID: 10934317 Review.
Cited by
-
Three cotton genes preferentially expressed in flower tissues encode actin-depolymerizing factors which are involved in F-actin dynamics in cells.J Exp Bot. 2010;61(1):41-53. doi: 10.1093/jxb/erp280. J Exp Bot. 2010. PMID: 19861654 Free PMC article.
-
Distribution of G-actin is related to root hair growth of wheat.Ann Bot. 2006 Jul;98(1):49-55. doi: 10.1093/aob/mcl084. Epub 2006 May 4. Ann Bot. 2006. PMID: 16675602 Free PMC article.
-
Ectopic expression of an activated RAC in Arabidopsis disrupts membrane cycling.Mol Biol Cell. 2005 Apr;16(4):1913-27. doi: 10.1091/mbc.e04-07-0562. Epub 2005 Feb 9. Mol Biol Cell. 2005. PMID: 15703216 Free PMC article.
-
Exploring the Role of the Plant Actin Cytoskeleton: From Signaling to Cellular Functions.Int J Mol Sci. 2023 Oct 23;24(20):15480. doi: 10.3390/ijms242015480. Int J Mol Sci. 2023. PMID: 37895158 Free PMC article. Review.
-
Actin Cytoskeleton as Actor in Upstream and Downstream of Calcium Signaling in Plant Cells.Int J Mol Sci. 2019 Mar 20;20(6):1403. doi: 10.3390/ijms20061403. Int J Mol Sci. 2019. PMID: 30897737 Free PMC article. Review.
References
-
- Agnew, B.J., Minamide, L.S., and Bamburg, J.R. (1995). Reactivation of phosphorylated actin depolymerizing factor and identification of the regulatory site. J. Biol. Chem. 270, 17582–17587. - PubMed
-
- Allwood, E.G., Smertenko, A.P., and Hussey, P.J. (2001). Phosphorylation of plant actin-depolymerizing factor by calmodulin-like domain protein kinase. FEBS Lett. 499, 97–100. - PubMed
-
- Arabidopsis Genome Initiative (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796–815. - PubMed
-
- Arber, S., Barbayannis, F.A., Hanser, H., Schindler, C., Stanton, C.A., Bernard, O., and Caroni, P. (1998). Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393, 805–809. - PubMed
-
- Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., and Struhl, K. (2000). Current Protocols in Molecular Biology. (New York: John Wiley & Sons).
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

