The transcription factors HvABI5 and HvVP1 are required for the abscisic acid induction of gene expression in barley aleurone cells
- PMID: 12509536
- PMCID: PMC143496
- DOI: 10.1105/tpc.007096
The transcription factors HvABI5 and HvVP1 are required for the abscisic acid induction of gene expression in barley aleurone cells
Abstract
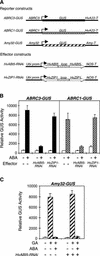
The abscisic acid (ABA) response promoter complexes (ABRCs) of the HVA1 and HVA22 genes have been shown to confer ABA-induced gene expression in cereals. A barley basic domain/Leu zipper (bZIP) transcription factor, HvABI5, is able to recognize ABRCs in vitro in a sequence-specific manner and to transactivate ABRC-beta-glucuronidase reporter genes when introduced to barley aleurone cells via particle bombardment. This transactivation is dependent on the presence of another transcription factor, HvVP1, and cannot be blocked by the negative regulator abi1-1. Using the double-stranded RNA interference technique, we show that HvABI5 and HvVP1 are necessary for the ABA induction of gene expression but have no effect on another hormone-regulated process, the gibberellin-induced and ABA-suppressed expression of alpha-amylase. Our work indicates that although other typical plant bZIP transcription factors may bind ABRCs in vitro, HvABI5 is related to a subfamily of bZIPs responsible for the ABA induction of gene expression. Furthermore, HvABI5 and HvVP1 are not involved in the ABA suppression of gene expression.
Figures







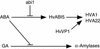
References
-
- Bray, E.A. (1997). Plant responses to water deficit. Trends Plant Sci. 2, 48–54.
-
- Busk, P.K., and Pages, M. (1998). Regulation of abscisic acid-induced transcription. Plant Mol. Biol. 37, 425–435. - PubMed
-
- Carles, C., Bies-Etheve, N., Aspart, L., Leon-Kloosterziel, K.M., Koornneef, M., Echeverria, M., and Delseny, M. (2002). Regulation of Arabidopsis thaliana Em genes: Role of ABI5. Plant J. 30, 373–383. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

