Characterization of a cellulase containing a family 30 carbohydrate-binding module (CBM) derived from Clostridium thermocellum CelJ: importance of the CBM to cellulose hydrolysis
- PMID: 12511497
- PMCID: PMC145318
- DOI: 10.1128/JB.185.2.504-512.2003
Characterization of a cellulase containing a family 30 carbohydrate-binding module (CBM) derived from Clostridium thermocellum CelJ: importance of the CBM to cellulose hydrolysis
Abstract
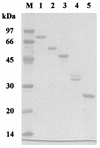
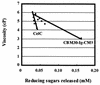
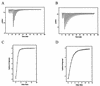
Clostridium thermocellum CelJ is a modular enzyme containing a family 30 carbohydrate-binding module (CBM) and a family 9 catalytic module at its N-terminal moiety. To investigate the functions of the CBM and the catalytic module, truncated derivatives of CelJ were constructed and characterized. Isothermal titration calorimetric studies showed that the association constants (K(a)) of the CBM polypeptide (CBM30) for the binding of cellopentaose and cellohexaose were 1.2 x 10(4) and 6.4 x 10(4) M(-1), respectively, and that the binding of CBM30 to these ligands is enthalpically driven. Qualitative analyses showed that CBM30 had strong affinity for cellulose and beta-1,3-1,4-mixed glucan such as barley beta-glucan and lichenan. Analyses of the hydrolytic action of the enzyme comprising the CBM and the catalytic module showed that the enzyme is a processive endoglucanse with strong activity towards carboxymethylcellulose, barley beta-glucan and lichenan. By contrast, the catalytic module polypeptide devoid of the CBM showed negligible activity toward these substrates. These observations suggest that the CBM is extremely important not only because it mediates the binding of the enzyme to the substrates but also because it participates in the catalytic function of the enzyme or contributes to maintaining the correct tertiary structure of the family 9 catalytic module for expressing enzyme activity.
Figures


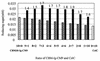
 , activity of CBM30-Ig-CM9;
, activity of CBM30-Ig-CM9;  , activity of CelC;
, activity of CelC;  , sum of the theoretical activities of respective enzymes; ▪, observed activity of the combined enzymes.
, sum of the theoretical activities of respective enzymes; ▪, observed activity of the combined enzymes.
References
-
- Ali, B. R., L. Zhou, F. M. Graves, R. B. Freedman, G. W. Black, H. J. Gilbert, and G. P. Hazelwood. 1995. Cellulases and hemicellulases of the anaerobic fungus Piromyces constitute a multiprotein cellulose-binding complex and are encoded by multigene families. FEMS Microbiol. Lett. 125:15-21. - PubMed
-
- Ali, M. K., H. Hayashi, S. Karita, M. Goto, T. Kimura, K. Sakka, and K. Ohmiya. 2001. Importance of the carbohydrate-binding module of Clostridium stercorarium Xyn10B to xylan hydrolysis. Biosci. Biotechnol. Biochem. 65:41-47. - PubMed
-
- Arai, T., H. Ohara, T. Karita, T. Kimura, K. Sakka, and K. Ohmiya. 2001. Sequence of celQ and properties of CelQ, a component of the Clostridium thermocellum cellulosome. Appl. Microbiol. Biotechnol. 57:660-666. - PubMed
-
- Beguin, P., and P. M. Alzari. 1998. The cellulosome of Clostridium thermocellum. Biochem. Soc. Trans. 26:178-185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

