Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration
- PMID: 12511590
- PMCID: PMC151835
- DOI: 10.1172/JCI16157
Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration
Abstract
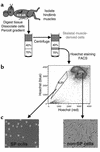
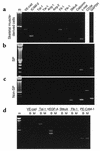
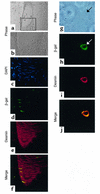
Vascular progenitors were previously isolated from blood and bone marrow; herein, we define the presence, phenotype, potential, and origin of vascular progenitors resident within adult skeletal muscle. Two distinct populations of cells were simultaneously isolated from hindlimb muscle: the side population (SP) of highly purified hematopoietic stem cells and non-SP cells, which do not reconstitute blood. Muscle SP cells were found to be derived from, and replenished by, bone marrow SP cells; however, within the muscle environment, they were phenotypically distinct from marrow SP cells. Non-SP cells were also derived from marrow stem cells and contained progenitors with a mesenchymal phenotype. Muscle SP and non-SP cells were isolated from Rosa26 mice and directly injected into injured muscle of genetically matched recipients. SP cells engrafted into endothelium during vascular regeneration, and non-SP cells engrafted into smooth muscle. Thus, distinct populations of vascular progenitors are resident within skeletal muscle, are derived from bone marrow, and exhibit different cell fates during injury-induced vascular regeneration.
Figures



Comment in
-
Machinations of the marrow.J Clin Invest. 2003 Jan;111(1):29-30. doi: 10.1172/JCI17546. J Clin Invest. 2003. PMID: 12511584 Free PMC article. Review. No abstract available.
References
-
- Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Shimizu K, et al. Host bone marrow cells are a source of donor intimal smooth muscle-like cells in murine aortic transplant arteriopathy. Nat. Med. 2001;7:738–741. - PubMed
-
- Orlic D, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. - PubMed

