Entry of viruses through the epithelial barrier: pathogenic trickery
- PMID: 12511869
- PMCID: PMC7097689
- DOI: 10.1038/nrm1005
Entry of viruses through the epithelial barrier: pathogenic trickery
Abstract
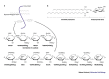
Mucosal surfaces--such as the lining of the gut or the reproductive tract--are the main point of entry for viruses into the body. As such, almost all viruses interact with epithelial cells, and make use of the normal epithelial signalling and trafficking pathways of the host cell. In addition to protein receptors, carbohydrate chains of proteoglycans and epithelial-membrane glycosphingolipids have emerged as a new class of receptors for viral attachment to the host cell.
Figures


References
-
- Gabius H, Andre S, Kaltner H, Siebert H. The sugar code: functional lectinomics. Biochim. Biophys. Acta. 2002;19:2–3. - PubMed
-
- Compans RW. Virus entry and release in polarized epithelial cells. Curr. Top. Microbiol. Immunol. 1995;202:209–219. - PubMed
-
- Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nature Med. 1997;3:42–47. - PubMed
-
- Meng G, et al. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nature Med. 2002;8:150–156. - PubMed
-
- Smith GA, Enquist LW. Break ins and break outs: viral interactions with the cytoskeleton of mammalian cells. Annu. Rev. Cell Dev. Biol. 2002;18:135–161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

