Hydrogenotrophic methanogenesis by moderately acid-tolerant methanogens of a methane-emitting acidic peat
- PMID: 12513979
- PMCID: PMC152423
- DOI: 10.1128/AEM.69.1.74-83.2003
Hydrogenotrophic methanogenesis by moderately acid-tolerant methanogens of a methane-emitting acidic peat
Abstract
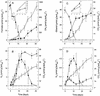
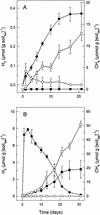
The emission of methane (1.3 mmol of CH(4) m(-2) day(-1)), precursors of methanogenesis, and the methanogenic microorganisms of acidic bog peat (pH 4.4) from a moderately reduced forest site were investigated by in situ measurements, microcosm incubations, and cultivation methods, respectively. Bog peat produced CH(4) (0.4 to 1.7 micro mol g [dry wt] of soil(-1) day(-1)) under anoxic conditions. At in situ pH, supplemental H(2)-CO(2), ethanol, and 1-propanol all increased CH(4) production rates while formate, acetate, propionate, and butyrate inhibited the production of CH(4); methanol had no effect. H(2)-dependent acetogenesis occurred in H(2)-CO(2)-supplemented bog peat only after extended incubation periods. Nonsupplemented bog peat initially produced small amounts of H(2) that were subsequently consumed. The accumulation of H(2) was stimulated by ethanol and 1-propanol or by inhibiting methanogenesis with bromoethanesulfonate, and the consumption of ethanol was inhibited by large amounts of H(2); these results collectively indicated that ethanol- or 1-propanol-utilizing bacteria were trophically associated with H(2)-utilizing methanogens. A total of 10(9) anaerobes and 10(7) hydrogenotrophic methanogens per g (dry weight) of bog peat were enumerated by cultivation techniques. A stable methanogenic enrichment was obtained with an acidic, H(2)-CO(2)-supplemented, fatty acid-enriched defined medium. CH(4) production rates by the enrichment were similar at pH 4.5 and 6.5, and acetate inhibited methanogenesis at pH 4.5 but not at pH 6.5. A total of 27 different archaeal 16S rRNA gene sequences indicative of Methanobacteriaceae, Methanomicrobiales, and Methanosarcinaceae were retrieved from the highest CH(4)-positive serial dilutions of bog peat and methanogenic enrichments. A total of 10 bacterial 16S rRNA gene sequences were also retrieved from the same dilutions and enrichments and were indicative of bacteria that might be responsible for the production of H(2) that could be used by hydrogenotrophic methanogens. These results indicated that in this acidic bog peat, (i) H(2) is an important substrate for acid-tolerant methanogens, (ii) interspecies hydrogen transfer is involved in the degradation of organic carbon, (iii) the accumulation of protonated volatile fatty acids inhibits methanogenesis, and (iv) methanogenesis might be due to the activities of methanogens that are phylogenetic members of the Methanobacteriaceae, Methanomicrobiales, and Methanosarcinaceae.
Figures



References
-
- Aselmann, I., and P. J. Crutzen. 1989. Global distribution of natural freshwater wetlands and rice paddies, their net primary productivity, seasonality and possible methane emission. J. Atmos. Chem. 8:307-359.
-
- Boone, R. B., W. B. Whitman, and P. Rouviere. 1993. Diversity and taxonomy of methanogens, p. 35-80. In J. G. Ferry (ed.), Methanogenesis. Chapman & Hall, New York, N.Y.
-
- Bryant, M. P., and D. R. Boone. 1987. Emended description of strain MST (DSM 800T), the type strain of Methanosarcina barkeri. Int. J. Syst. Bacteriol. 37:169-170.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

