Degradation of anthracene by Mycobacterium sp. strain LB501T proceeds via a novel pathway, through o-phthalic acid
- PMID: 12513994
- PMCID: PMC152392
- DOI: 10.1128/AEM.69.1.186-190.2003
Degradation of anthracene by Mycobacterium sp. strain LB501T proceeds via a novel pathway, through o-phthalic acid
Erratum in
- Appl Environ Microbiol. 2003 May;69(5):3026
Abstract
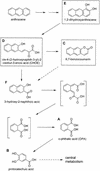
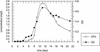
Mycobacterium sp. strain LB501T utilizes anthracene as a sole carbon and energy source. We analyzed cultures of the wild-type strain and of UV-generated mutants impaired in anthracene utilization for metabolites to determine the anthracene degradation pathway. Identification of metabolites by comparison with authentic standards and transient accumulation of o-phthalic acid by the wild-type strain during growth on anthracene suggest a pathway through o-phthalic acid and protocatechuic acid. As the only productive degradation pathway known so far for anthracene proceeds through 2,3-dihydroxynaphthalene and the naphthalene degradation pathway to form salicylate, this indicates the existence of a novel anthracene catabolic pathway in Mycobacterium sp. LB501T.
Figures
References
-
- Akhtar, M. N., D. R. Boyd, N. J. Thompson, M. Koreeda, D. T. Gibson, V. Mahadevan, and D. M. Jerina. 1975. Absolute stereochemistry of the dihydroanthracene-cis- and -trans-1,2-diols produced from anthracene by mammals and bacteria. J.C.S. Perkin I:2506-2511. - PubMed
-
- Cerniglia, C. E. 1984. Microbial metabolism of polycyclic aromatic hydrocarbons. Adv. Appl. Microbiol. 30:31-71. - PubMed
-
- Dean-Ross, D., J. D. Moody, J. P. Freeman, D. R. Doerge, and C. E. Cerniglia. 2001. Metabolism of anthracene by a Rhodococcus species. FEMS Microbiol. Lett. 204:205-211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

