Carbon and hydrogen isotopic fractionation during anaerobic biodegradation of benzene
- PMID: 12513995
- PMCID: PMC152413
- DOI: 10.1128/AEM.69.1.191-198.2003
Carbon and hydrogen isotopic fractionation during anaerobic biodegradation of benzene
Abstract
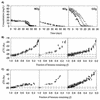
Compound-specific isotope analysis has the potential to distinguish physical from biological attenuation processes in the subsurface. In this study, carbon and hydrogen isotopic fractionation effects during biodegradation of benzene under anaerobic conditions with different terminal-electron-accepting processes are reported for the first time. Different enrichment factors (epsilon ) for carbon (range of -1.9 to -3.6 per thousand ) and hydrogen (range of -29 to -79 per thousand ) fractionation were observed during biodegradation of benzene under nitrate-reducing, sulfate-reducing, and methanogenic conditions. These differences are not related to differences in initial biomass or in rates of biodegradation. Carbon isotopic enrichment factors for anaerobic benzene biodegradation in this study are comparable to those previously published for aerobic benzene biodegradation. In contrast, hydrogen enrichment factors determined for anaerobic benzene biodegradation are significantly larger than those previously published for benzene biodegradation under aerobic conditions. A fundamental difference in the previously proposed initial step of aerobic versus proposed anaerobic biodegradation pathways may account for these differences in hydrogen isotopic fractionation. Potentially, C-H bond breakage in the initial step of the anaerobic benzene biodegradation pathway may account for the large fractionation observed compared to that in aerobic benzene biodegradation. Despite some differences in reported enrichment factors between cultures with different terminal-electron-accepting processes, carbon and hydrogen isotope analysis has the potential to provide direct evidence of anaerobic biodegradation of benzene in the field.
Figures

References
-
- Ahad, J. M. E., B. Sherwood Lollar, E. A. Edwards, G. F. Slater, and B. E. Sleep. 2000. Carbon isotope fractionation during anaerobic biodegradation of toluene: implications for intrinsic bioremediation. Environ. Sci. Technol. 34:892-896.
-
- Bloom, Y., R. Aravena, D. Hunkeler, E. Edwards, and S. K. Frape. 2000. Carbon isotope fractionation during microbial dechlorination of trichloroethene, cis-1,2-dichloroethene, and vinyl chloride: implications for assessment of natural attenuation. Environ. Sci. Technol. 34:2768-2772.
-
- Borden, R. C., C. A. Gomez, and M. T. Becker. 1995. Geochemical indicators of intrinsic bioremediation. Ground Water 33:180-189.
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

