EGF-CFC proteins are essential coreceptors for the TGF-beta signals Vg1 and GDF1
- PMID: 12514096
- PMCID: PMC195969
- DOI: 10.1101/gad.1041203
EGF-CFC proteins are essential coreceptors for the TGF-beta signals Vg1 and GDF1
Abstract
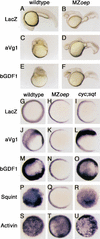
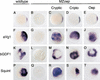
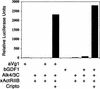
The TGF-beta signals Nodal, Activin, GDF1, and Vg1 have been implicated in mesoderm induction and left-right patterning. Nodal and Activin both activate Activin receptors, but only Nodal requires EGF-CFC coreceptors for signaling. We report that Vg1 and GDF1 signaling in zebrafish also depends on EGF-CFC proteins, but not on Nodal signals. Correspondingly, we find that in Xenopus Vg1 and GDF1 bind to and signal through Activin receptors only in the presence of EGF-CFC proteins. These results establish that multiple TGF-beta signals converge on Activin receptor/EGF-CFC complexes and suggest a more widespread requirement for coreceptors in TGF-beta signaling than anticipated previously.
Figures
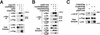
References
-
- Bamford RN, Roessler E, Burdine RD, Saplakoglu U, dela Cruz J, Splitt M, Goodship JA, Towbin J, Bowers P, Ferrero GB, et al. Loss-of-function mutations in the EGF-CFC gene CFC1 are associated with human left-right laterality defects. Nat Genet. 2000;26:365–369. - PubMed
-
- Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. - PubMed
-
- Ding J, Yang L, Yan YT, Chen A, Desai N, Wynshaw-Boris A, Shen MM. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature. 1998;395:702–707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
