Multipotent cell lineages in early mouse development depend on SOX2 function
- PMID: 12514105
- PMCID: PMC195970
- DOI: 10.1101/gad.224503
Multipotent cell lineages in early mouse development depend on SOX2 function
Abstract
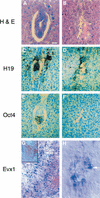
Each cell lineage specified in the preimplantation mammalian embryo depends on intrinsic factors for its development, but there is also mutual interdependence between them. OCT4 is required for the ICM/epiblast lineage, and at transient high levels for extraembryonic endoderm, but also indirectly through its role in regulating Fgf4 expression, for the establishment and proliferation of extraembryonic ectoderm from polar trophectoderm. The transcription factor SOX2 has also been implicated in the regulation of Fgf4 expression. We have used gene targeting to inactivate Sox2, examining the phenotypic consequences in mutant embryos and in chimeras in which the epiblast is rescued with wild-type ES cells. We find a cell-autonomous requirement for the gene in both epiblast and extraembryonic ectoderm, the multipotent precursors of all embryonic and trophoblast cell types, respectively. However, an earlier role within the ICM may be masked by the persistence of maternal protein, whereas the lack of SOX2 only becomes critical in the chorion after 7.5 days postcoitum. Our data suggest that maternal components could be involved in establishing early cell fate decisions and that a combinatorial code, requiring SOX2 and OCT4, specifies the first three lineages present at implantation.
Figures



References
-
- Ambrosetti DC, Schöler HR, Dailey L, Basilico C. Modulation of the activity of multiple transcriptional activation domains by the HMG and POU domains mediates the synergistic action of Sox2 and Oct-3 on the FGF-4 enhancer. J Biol Chem. 2000;275:23387–23397. - PubMed
-
- Avilion AA, Bell DM, Lovell-Badge R. Micro-capillary tube in situ hybridisation: A novel method for processing small individual samples. Genesis. 2000;27:76–80. - PubMed
-
- Beddington RSP, Robertson EJ. An assessment of the developmental potential of embryonic stem cells in the midgestation embryo. Development. 1989;105:733–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
