Actin-ATP hydrolysis is a major energy drain for neurons
- PMID: 12514193
- PMCID: PMC6742122
- DOI: 10.1523/JNEUROSCI.23-01-00002.2003
Actin-ATP hydrolysis is a major energy drain for neurons
Abstract
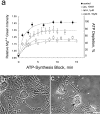
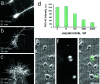
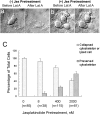
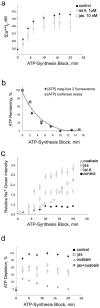
In cultured chick ciliary neurons, when ATP synthesis is inhibited, ATP depletion is reduced approximately 50% by slowing actin filament turnover with jasplakinolide or latrunculin A. Jasplakinolide inhibits actin disassembly, and latrunculin A prevents actin assembly by sequestering actin monomers. Cytochalasin D, which allows assembly-disassembly, but only at pointed ends, is less effective in conserving ATP. Ouabain, an Na(+)-K(+)-ATPase inhibitor, and jasplakinolide both prevent approximately 50% of the ATP loss. When applied together, they completely prevent ATP loss over a period of 20 min, suggesting that filament stabilization reduces ATP consumption by decreasing actin-ATP hydrolysis directly rather than indirectly by modulating the activity of Na(+)-K(+)-ATPase, a major energy consumer.
Figures
References
-
- Alberts B, Bray D, Raff M, Roberts K, Watson JD. Molecular biology of the cell, pp 41–88. Garland; London: 1994.
-
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. - PubMed
-
- Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
