Opposite action of beta1- and beta2-adrenergic receptors on Ca(V)1 L-channel current in rat adrenal chromaffin cells
- PMID: 12514203
- PMCID: PMC6742137
- DOI: 10.1523/JNEUROSCI.23-01-00073.2003
Opposite action of beta1- and beta2-adrenergic receptors on Ca(V)1 L-channel current in rat adrenal chromaffin cells
Abstract
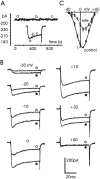
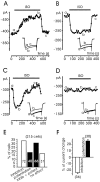
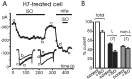
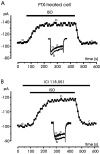
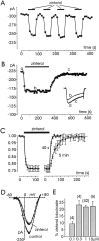
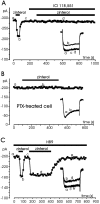
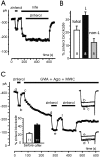
Voltage-gated Ca(2+) channels of chromaffin cells are modulated by locally released neurotransmitters through autoreceptor-activated G-proteins. Clear evidence exists in favor of a Ca(2+) channel gating inhibition mediated by purinergic, opioidergic, and alpha-adrenergic autoreceptors. Few and contradictory data suggest also a role of beta-adrenergic autoreceptors (beta-ARs), the action of which, however, remains obscure. Here, using patch-perforated recordings, we show that rat chromaffin cells respond to the beta-AR agonist isoprenaline (ISO) by either upmodulating or downmodulating the amplitude of Ca(2+) currents through two distinct modulatory pathways. ISO (1 microm) could cause either fast inhibition (approximately 25%) or slow potentiation (approximately 25%), or a combination of the two actions. Both effects were completely prevented by propranolol. Slow potentiation was more evident in cells pretreated with pertussis toxin (PTX) or when beta(1)-ARs were selectively stimulated with ISO + ICI118,551. Potentiation was absent when the beta(2)-AR-selective agonist zinterol (1 microm), the protein kinase A (PKA) inhibitor H89, or nifedipine was applied, suggesting that potentiation is associated with a PKA-mediated phosphorylation of L-channels (approximately 40% L-current increase) through beta(1)-ARs. The ISO-induced inhibition was fast and reversible, preserved in cell treated with H89, and mimicked by zinterol. The action of zinterol was mostly on L-channels (38% inhibition). Zinterol action preserved the channel activation kinetics, the voltage-dependence of the I-V characteristic, and was removed by PTX, suggesting that beta(2)AR-mediated channel inhibition was mainly voltage independent and coupled to G(i)/G(o)-proteins. Sequential application of zinterol and ISO mimicked the dual action (inhibition/potentiation) of ISO alone. The two kinetically and pharmacologically distinct beta-ARs signaling uncover alternative pathways, which may serve the autocrine control of Ca(2+)-dependent exocytosis and other related functions of rat chromaffin cells.
Figures




References
-
- Albillos A, Carbone E, Gandía L, García AG, Pollo A. Opioid inhibition of Ca2+ channel subtypes in bovine chromaffin cells: selectivity of action and voltage-dependence. Eur J Neurosci. 1996b;8:1561–1570. - PubMed
-
- Artalejo CR, Ariano MA, Perlman RA, Fox AP. Activation of facilitation calcium channels in chromaffin cells by D1 dopamine receptors through a cAMP/protein kinase A-dependent mechanism. Nature. 1990;348:239–242. - PubMed
-
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991;121:101–117. - PubMed
-
- Bean BP, Nowycky MC, Tsien RW. β-Adrenergic modulation of calcium channels in frog ventricular heart cells. Nature. 1984;307:371–375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
