KIF17 dynamics and regulation of NR2B trafficking in hippocampal neurons
- PMID: 12514209
- PMCID: PMC6742138
- DOI: 10.1523/JNEUROSCI.23-01-00131.2003
KIF17 dynamics and regulation of NR2B trafficking in hippocampal neurons
Abstract
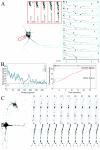
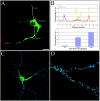
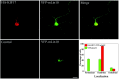
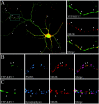
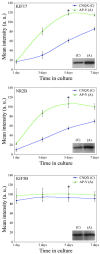
KIF17, a recently characterized member of the kinesin superfamily proteins, has been proposed to bind in vitro to a protein complex containing mLin10 (Mint1/X11) and the NR2B subunit of the NMDA receptors (NMDARs). In the mammalian brain, NMDARs play an important role in synaptic plasticity, learning, and memory. Here we present, for the first time, the dynamic properties of KIF17 and provide evidence of its function in the transport of NR2B in living mammalian neurons. KIF17 vesicles enter and move specifically along dendrites in a processive way, at an average speed of 0.76 microm/sec. These vesicles are effectively associated with extrasynaptic NR2B, and thus they transport and deliver NR2B subunits in dendrites. However, KIF17 does not seem to enter directly into postsynaptic regions. Cellular knockdown or functional blockade of KIF17 significantly impairs NR2B expression and its synaptic localization. Interestingly, the decrease in the number of synaptic NR2B subunits is followed by a parallel increase in the number of NR2A subunits at synapses. In contrast, upregulation of the expression level of NR2B, after treatment with the NMDAR antagonist D(-)-2-amino-5-phosphonopentanoic acid, simultaneously increases the expression level of KIF17. These observations concerning the downregulation or upregulation of KIF17 and NR2B reveal the probable existence of a shared regulation process between the motor and its cargo. Taken together, these results illustrate the complex mechanisms underlying the active transport and regulation of NR2B by the molecular motor KIF17 in living hippocampal neurons.
Figures

References
-
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. - PubMed
-
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
-
- Burack MA, Silverman MA, Banker G. The role of selective transport in neuronal protein sorting. Neuron. 2000;26:465–472. - PubMed
-
- Craven SE, El-Husseini AE, Bredt DS. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron. 1999;22:497–509. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
