Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring
- PMID: 12514227
- PMCID: PMC6742135
- DOI: 10.1523/JNEUROSCI.23-01-00297.2003
Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring
Abstract
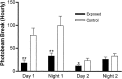
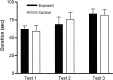
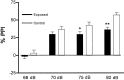
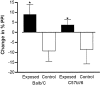
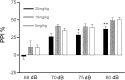
Maternal viral infection is known to increase the risk for schizophrenia and autism in the offspring. Using this observation in an animal model, we find that respiratory infection of pregnant mice (both BALB/c and C57BL/6 strains) with the human influenza virus yields offspring that display highly abnormal behavioral responses as adults. As in schizophrenia and autism, these offspring display deficits in prepulse inhibition (PPI) in the acoustic startle response. Compared with control mice, the infected mice also display striking responses to the acute administration of antipsychotic (clozapine and chlorpromazine) and psychomimetic (ketamine) drugs. Moreover, these mice are deficient in exploratory behavior in both open-field and novel-object tests, and they are deficient in social interaction. At least some of these behavioral changes likely are attributable to the maternal immune response itself. That is, maternal injection of the synthetic double-stranded RNA polyinosinic-polycytidylic acid causes a PPI deficit in the offspring in the absence of virus. Therefore, maternal viral infection has a profound effect on the behavior of adult offspring, probably via an effect of the maternal immune response on the fetus.
Figures


References
-
- Boin F, Zanardini R, Pioli R, Altamura CA, Maes M, Germarelli M. Association between -G308A tumor necrosis factor alpha gene polymorphism and schizophrenia. Mol Psychiat. 2001;6:79–82. - PubMed
-
- Brown AS, Schaefer CA, Wyatt RJ, Goetz R, Begg MD, Gorman JM, Susser ES. Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: a prospective birth cohort study. Schizophrenia Bull. 2000;26:287–295. - PubMed
-
- Burger RA, Warren RP. Possible immunogenetic basis for autism. Ment Retard Dev Disabil Res Rev. 1998;4:137–141.
-
- Ciaranello AL, Ciaranello RD. The neurobiology of infantile autism. Annu Rev Neurosci. 1995;18:101–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
