Mycobacteria target DC-SIGN to suppress dendritic cell function
- PMID: 12515809
- PMCID: PMC2193797
- DOI: 10.1084/jem.20021229
Mycobacteria target DC-SIGN to suppress dendritic cell function
Abstract
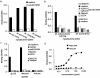
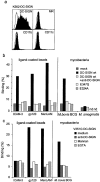
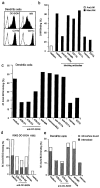
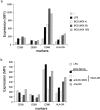
Mycobacterium tuberculosis represents a world-wide health risk and immunosuppression is a particular problem in M. tuberculosis infections. Although macrophages are primarily infected, dendritic cells (DCs) are important in inducing cellular immune responses against M. tuberculosis. We hypothesized that DCs represent a target for M. tuberculosis and that the observed immuno-suppression results from modulation of DC functions. We demonstrate that the DC-specific C-type lectin DC-SIGN is an important receptor on DCs that captures and internalizes intact Mycobacterium bovis bacillus Calmette-Guérin (BCG) through the mycobacterial cell wall component ManLAM. Antibodies against DC-SIGN block M. bovis BCG infection of DCs. ManLAM is also secreted by M. tuberculosis-infected macrophages and has been implicated as a virulence factor. Strikingly, ManLAM binding to DC-SIGN prevents mycobacteria- or LPS-induced DC maturation. Both mycobacteria and LPS induce DC maturation through Toll-like receptor (TLR) signaling, suggesting that DC-SIGN, upon binding of ManLAM, interferes with TLR-mediated signals. Blocking antibodies against DC-SIGN reverse the ManLAM-mediated immunosuppressive effects. Our results suggest that M. tuberculosis targets DC-SIGN both to infect DCs and to down-regulate DC-mediated immune responses. Moreover, we demonstrate that DC-SIGN has a broader pathogen recognition profile than previously shown, suggesting that DC-SIGN may represent a molecular target for clinical intervention in infections other than HIV-1.
Figures



Comment in
-
A dangerous liaison between two major killers: Mycobacterium tuberculosis and HIV target dendritic cells through DC-SIGN.J Exp Med. 2003 Jan 6;197(1):1-5. doi: 10.1084/jem.20021964. J Exp Med. 2003. PMID: 12515808 Free PMC article. No abstract available.
References
-
- Demangel, C., and W.J. Britton. 2000. Interaction of dendritic cells with mycobacteria: where the action starts. Immunol. Cell Biol. 78:318–324. - PubMed
-
- Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. - PubMed
-
- Prigozy, T.I., P.A. Sieling, D. Clemens, P.L. Stewart, S.M. Behar, S.A. Porcelli, M.B. Brenner, R.L. Modlin, and M. Kronenberg. 1997. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity. 6:187–197. - PubMed
-
- Manabe, Y.C., and W.R. Bishai. 2000. Latent Mycobacterium tuberculosis-persistence, patience, and winning by waiting. Nat. Med. 6:1327–1329. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

