Molecular assays for detection of human metapneumovirus
- PMID: 12517833
- PMCID: PMC149567
- DOI: 10.1128/JCM.41.1.100-105.2003
Molecular assays for detection of human metapneumovirus
Abstract
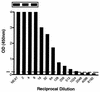
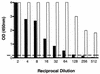
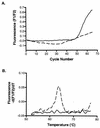
The recent description of the respiratory pathogen human metapneumovirus (hMPV) has highlighted a deficiency in current diagnostic techniques for viral agents associated with acute lower respiratory tract infections. We describe two novel approaches to the detection of viral RNA by use of reverse transcriptase PCR (RT-PCR). The PCR products were identified after capture onto a solid-phase medium by hybridization with a sequence-specific, biotinylated oligonucleotide probe. The assay was applied to the screening of 329 nasopharyngeal aspirates sampled from patients suffering from respiratory tract disease. These samples were negative for other common microbial causes of respiratory tract disease. We were able to detect hMPV sequences in 32 (9.7%) samples collected from Australian patients during 2001. To further reduce result turnaround times we designed a fluorogenic TaqMan oligoprobe and combined it with the existing primers for use on the LightCycler platform. The real-time RT-PCR proved to be highly reproducible and detected hMPV in an additional 6 out of 62 samples (9.6%) tested during the comparison of the two diagnostic approaches. We found the real-time RT-PCR to be the test of choice for future investigation of samples for hMPV due to its speed, reproducibility, specificity, and sensitivity.
Figures


References
-
- Berg, J., V. Nagl, G. Mühlbauer, and H. Stekel. 2001. Single-tube two-round polymerase chain reaction using the LightCycler instrument. J. Clin. Virol. 20:71-75. - PubMed
-
- Collins, P. L., K. McIntosh, and R. M. Chanock. 1996. Respiratory syncytial virus, p. 1313-1351. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven, Philadelphia, Pa.
-
- Crockett, A. O., and C. T. Wittwer. 2001. Fluorescein-labelled oligonucleotides for real-time PCR: using the inherent quenching of deoxyguanosine nucleotides. Anal. Biochem. 290:89-97. - PubMed
-
- Holberg, C. J., A. L. Wright, F. D. Martinez, C. G. Ray, L. M. Taussig, and M. D. Lebowitz. 1991. Risk factors for respiratory syncytial virus-induced lower respiratory illnesses in the first year of life. Am. J. Epidemiol. 133:1135-1151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

