Comparison of LightCycler PCR, rapid antigen immunoassay, and culture for detection of group A streptococci from throat swabs
- PMID: 12517855
- PMCID: PMC149598
- DOI: 10.1128/JCM.41.1.242-249.2003
Comparison of LightCycler PCR, rapid antigen immunoassay, and culture for detection of group A streptococci from throat swabs
Abstract
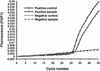
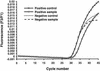
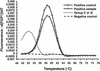
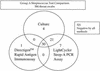
We compared the performance characteristics of a real-time PCR method, the LightCycler Strep-A assay (Roche Applied Science, Indianapolis, Ind.), to those of a rapid antigen immunoassay, the Directigen 1-2-3 Group A Strep Test kit (BD Diagnostic Systems, Sparks, Md.), and a standard culture method for detection of group A streptococci (GAS) from 384 throat swabs. The LightCycler PCR produced more positive results (n = 58) than either culture (n = 55) or the Directigen immunoassay (n = 31). The results of the LightCycler PCR and the Directigen method were independently compared to the results of the accepted "gold standard," bacterial culture. The sensitivities, specificities, and positive and negative predictive values for this comparison were as follows: for the Directigen method, 55, 99, 97, and 93%, respectively; for the LightCycler PCR, 93, 98, 88, and 99%, respectively. In no case was a throat swab positive by both the LightCycler PCR and the Directigen method but negative by culture. The medical histories of patients whose throat swabs were negative by culture but positive by either the LightCycler PCR (n = 7) or the Directigen method (n = 1) were reviewed. All of these patients had signs or symptoms compatible with GAS disease, and therefore, all of these discordant positive results (along with positive results by either the Directigen method or the LightCycler PCR that agreed with the culture results) were counted as true positives for statistical analysis. For this analysis, the LightCycler PCR detected more true-positive results than the culture method (58 versus 55 swabs); however, this difference was not statistically significant (P = 0.5465). In contrast, statistically significantly more true-positive results occurred by culture than by the Directigen method (55 versus 31 swabs; P < 0.0001) and by the LightCycler PCR than by the Directigen method (58 versus 31 swabs; P < 0.0001). The LightCycler PCR is a suitable stand-alone method for the detection of GAS from throat swabs. Additionally, this method requires less than half the personnel time and the procedure can be completed in considerably less time ( approximately 1 h) than our standard approach (up to 2 days) for detection of GAS in throat swabs (i.e., testing by the Directigen method with negative results verified by culture).
Figures


References
-
- American Academy of Pediatrics. 2000. Group A streptococcal infections, p. 526-536. In L. K. Pickering (ed.), 2000 Redbook; report of the Committee on Infectious Diseases, 25th ed. American Academy of Pediatrics, Elk Grove Village, Ill.
-
- Bergeron, M. G., D. Ke, C. Menard, F. J. Picard, M. Gagnon, M. Bernier, M. Ouellette, R. H. Roy, S. Marcoux, and W. D. Fraser. 2000. Rapid detection of group B streptococci in pregnant women after delivery. N. Engl. J. Med. 343:175-179. - PubMed
-
- Bisno, A. L. 2001. Acute pharyngitis. N. Engl. J. Med. 344:205-211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

