Na+/K+-pump ligands modulate gating of palytoxin-induced ion channels
- PMID: 12518045
- PMCID: PMC141024
- DOI: 10.1073/pnas.0135849100
Na+/K+-pump ligands modulate gating of palytoxin-induced ion channels
Abstract
The Na+/K+ pump is a ubiquitous P-type ATPase that binds three cytoplasmic Na+ ions deep within its core where they are temporarily occluded before being released to the extracellular surface. The 3Na+/2K+ -exchange transport cycle is completed when two extracellular K+ ions bind and become temporarily occluded within the protein and subsequently released to the cytoplasm. Coupling of Na+ -ion occlusion to phosphorylation of the pump by ATP and of K+ -ion occlusion to its dephosphorylation ensure the vectorial nature of net transport. The occluded-ion conformations, with binding sites inaccessible from either side, represent intermediate states in these alternating-access descriptions of transport. They afford protection against potentially catastrophic effects of inadvertently allowing simultaneous access from both membrane sides. The marine toxin, palytoxin, converts Na+/K+ pumps into nonselective cation channels, possibly by disrupting the normal strict coupling between opening of one access pathway in the Na+/K+ ATPase and closing of the other. We show here that gating of the channels in palytoxin-bound Na+/K+ pumps in excised membrane patches is modulated by the pump's physiological ligands: cytoplasmic application of ATP promotes opening of the channels, and extracellular replacement of Na+ ions by K+ ions promotes closing of the channels. This suggests that, despite the presence of bound palytoxin, certain partial reactions of the normal Na+/K+ -transport cycle persist and remain capable of effecting the conformational changes that control access to the pump's cation-binding sites. These findings affirm the alternating-access model of ion pumps and offer the possibility of examining ion occlusion/deocclusion reactions in single pump molecules.
Figures


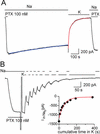

Comment in
-
From a pump to a pore: how palytoxin opens the gates.Proc Natl Acad Sci U S A. 2003 Jan 21;100(2):386-8. doi: 10.1073/pnas.0437775100. Epub 2003 Jan 13. Proc Natl Acad Sci U S A. 2003. PMID: 12525688 Free PMC article. No abstract available.
References
-
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer; 2001.
-
- Post R L, Hegyvary C, Kume S. J Biol Chem. 1972;247:6530–6540. - PubMed
-
- Beaugé L A, Glynn I M. Nature. 1979;280:510–512. - PubMed
-
- Patlak C S. Bull Math Biophys. 1957;19:209–235.
-
- Vidaver G A. J Theor Biol. 1966;10:301–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

