Asymmetry of the central apparatus defines the location of active microtubule sliding in Chlamydomonas flagella
- PMID: 12518061
- PMCID: PMC140907
- DOI: 10.1073/pnas.0135800100
Asymmetry of the central apparatus defines the location of active microtubule sliding in Chlamydomonas flagella
Abstract
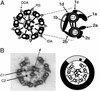
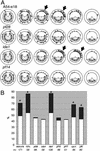
Regulation of ciliary and flagellar motility requires spatial control of dynein-driven microtubule sliding. However, the mechanism for regulating the location and symmetry of dynein activity is not understood. One hypothesis is that the asymmetrically organized central apparatus, through interactions with the radial spokes, transmits a signal to regulate dynein-driven microtubule sliding between subsets of doublet microtubules. Based on this model, we hypothesized that the orientation of the central apparatus defines positions of active microtubule sliding required to control bending in the axoneme. To test this, we induced microtubule sliding in axonemes isolated from wild-type and mutant Chlamydomonas cells, and then used electron microscopy to determine the orientation of the central apparatus. Transverse sections of wild-type axonemes revealed that the C1 microtubule is predominantly oriented toward the position of active microtubule sliding. In contrast, the central apparatus is randomly oriented in axonemes isolated from radial spoke deficient mutants. For outer arm dynein mutants, the C1 microtubule is oriented toward the position of active microtubule sliding in low calcium buffer, but is randomly oriented in high calcium buffer. These results provide evidence that the central apparatus defines the position of active microtubule sliding, and may regulate the size and shape of axonemal bends through interactions with the radial spokes. In addition, our results indicate that in high calcium conditions required to generate symmetric waveforms, the outer dynein arms are potential targets of the central pair-radial spoke control system.
Figures


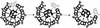
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

