Regular treatment with long acting beta agonists versus daily regular treatment with short acting beta agonists in adults and children with stable asthma
- PMID: 12519616
- PMCID: PMC6984628
- DOI: 10.1002/14651858.CD003901
Regular treatment with long acting beta agonists versus daily regular treatment with short acting beta agonists in adults and children with stable asthma
Abstract
Background: Selective beta-adrenergic agonists for use in asthma are: short acting (2-6 hours) and long acting (>12 hours). There has been little controversy about using short acting beta-agonists intermittently, but long acting beta-agonists are used regularly, and their regular use has been controversial.
Objectives: To determine the benefit or detriment of treatment with regular short- or long acting inhaled beta-agonists in chronic asthma.
Search strategy: A search was carried out using the Cochrane Airways Group register. Bibliographies of identified RCTs were searched for additional relevant RCTs. Authors of identified RCTs were contacted for other published and unpublished studies.
Selection criteria: All randomised studies of at least two weeks duration, comparing a long acting inhaled beta-agonist given twice daily with any short acting inhaled beta-agonist of equivalent bronchodilator effectiveness given regularly in chronic asthma.
Data collection and analysis: Two reviewers performed data extraction and study quality assessment independently. Authors of studies were contacted for missing data.
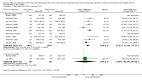
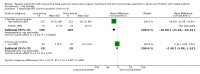
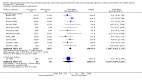
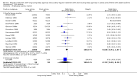
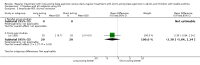
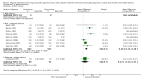
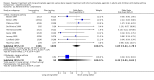
Main results: 31 studies met the inclusion criteria, 24 of parallel group and 7 cross over design. Salmeterol xinafoate was used as long acting agent in 22 studies and formoterol fumarate in 9. Salbutamol was the short acting agent used in 27 studies and terbutaline in 5. The treatment period was over 2 weeks in 29 studies, and at least 12 weeks in 20. 25 studies permitted a variety of co-intervention treatments, usually inhaled corticosteroid or cromones. One study did not permit inhaled corticosteroid. Long acting beta-agonists were significantly better than short acting for a variety of lung function measurements including morning PEF (Weighted Mean Difference (WMD) 33 l/min 95% CI 25, 42) or evening PEF (WMD 26 l/min 95% CI 18, 33); and had significantly lower scores for day and night time asthma symptom scores and percentage of days and nights without symptoms. They were also associated with a significantly lower use of rescue medication both during the day and night. Risk of exacerbations was not different between the two types of agent, but most studies were of short duration which limits the power to test for such differences.
Reviewer's conclusions: Long acting inhaled beta-agonists have advantages across a wide range of physiological and clinical outcomes for regular treatment.
Conflict of interest statement
E H Walters has taken part in collaborative clinical pharmacology studies with a number of pharmaceutical companies including GlaxoWellcome, Astra Zeneca, Pfizer, Boehringer, Schering Plough, SKB, and Novartis. He has, in the past, held consultancies with GlaxoWellcome, Pfizer and Zeneca. He has had sponsorship to meetings from a number of the companies listed over the past 15 years.
Figures
























































































Comment in
-
Review: regular inhaled long acting beta2 agonists are associated with better outcomes than short acting beta2 agonists in adults and children with chronic asthma.Evid Based Nurs. 2003 Jul;6(3):76. doi: 10.1136/ebn.6.3.76. Evid Based Nurs. 2003. PMID: 12882189 No abstract available.
References
References to studies included in this review
Beach 1993 {published data only (unpublished sought but not used)}
-
- Beach JR, Young CL, Harkawat R, Gardiner PV, Avery AJ, Coward GA, et al. Effect on airway responsiveness of six weeks treatment with salmeterol. Pulmonary Pharmacology 1993;6:155‐7. - PubMed
Bensch 2001 {published and unpublished data}
-
- Bensch G, Lapidus R, Levine B, Lumry W, Yegen U, Kiselev P, et al. A randomized, 12‐week, double‐blind, placebo‐controlled study comparing formoterol dry powder inhaler with albuterol metered‐dose inhaler. Annals of Allergy, Asthma, & Immunology 2001;86(1):19‐27. - PubMed
-
- Della Cioppa G, Kiseley P, Matos D, Yegen I, Townley RG. QID Albuterol worsens peak flow variability in asthma whereas BID Formoterol does not.(abstract). Annals of Allergy , Asthma & Immunology 1998;80:88.
Boulet 1997 {published data only (unpublished sought but not used)}
-
- Boulet LP, Laviolette M, Boucher S, et al. A Twelve week comparison of salmeterol and salbutamol in the treatment of mild to moderate asthma: a Canadian multicentre study. Journal of Allergy & Clinical Immunology 1997;99:13‐21. - PubMed
Britton 1992 {published and unpublished data}
-
- Britton MC, Earnshaw JS, Palmer JBD. A twelve month comparison of salmeterol with salbutamol in asthmatic patients. European Respiratory Journal 1992;5:1062‐67. - PubMed
-
- Fuller R. Safety of salmeterol in the treatment of asthma. European Respiratory Journal 1995;5:133‐7.
Byrnes 2000 {published data only (unpublished sought but not used)}
-
- Byrnes CA, Weber EA, Moorat A, Bush A. A Comparison of salmeterol 50mcg bd and 100mcg bd with salbutamol 200mcg qds in paediatric asthma. American Journal of Respiratory & Critical Care Medicine 1996;153(4):A408.
Castle 1993 {published data only}
D'Alonzo 1994 {published data only}
-
- Arledge T, Liddle D, Stahl E, Rossing T. Salmeterol does not cause tolerance during long term asthma therapy. Journal of Allergy & Clinical Immunology 1996;98:1116‐9. - PubMed
-
- D'Alonzo GE. Efficacy of inhaled salmeterol in the treatment of asthma. European Respiratory Review 1995;5:128‐32.
-
- D'Alonzo GE, Nathan RA, Henchowicz MD. Salmeterol xinafoate as maintenance therapy compared with albuterol in patients with asthma. The Journal of the American Medical Association 1994;271:1412‐6. - PubMed
-
- Nathan R, Seltzer J, Kemp J, Chervinsky P, Alexander W, Liddle R, et al. Safety of salmeterol in the maintenance treatment of asthma. Annals of Allergy, Asthma, & Immunology 1995;75:243‐8. - PubMed
-
- Pearlman DS. Long‐acting beta 2‐agonist salmeterol compared with albuterol in maintenance asthma therapy. Annals of Allergy, Asthma, & Immunology 1995;75:180‐4. - PubMed
De Carli 1995 {published and unpublished data}
-
- Carli G, Arpinelli F, Irvine SH, Bamfi F, Ravinetto R, Recchia G. Change of quality of life induced by salmeterol and salbutamol in adult asthmatic patients. Therapie 1995;50 Suppl:N118.
De Oliveira 1998 {published data only}
-
- Oliveira M, Jardim J, Faresin S, Lucas S, Nery L. Efficacy of and tolerance to salmeterol compared to salbutamol in patients with bronchial asthma. Revista da Associacao Medica Brasileira 1998;44:169‐75. - PubMed
Ekstrom 1998a {published data only}
-
- Ekstrom T, Ringdal N, Tukiainen P, Runnerstrom E, Soliman S. A 3 month comparison of formoterol with terbutaline via turbuhaler. A placebo controlled study. Annals of Allergy, Asthma, & Immunology 1998;81:225‐30. - PubMed
Ekstrom 1998b {published data only}
-
- Ekstrom T, Ringdal N, Sobradillo V, Runnerstrom E, Soliman S. Low‐dose formoterol Turbuhaler(TM) (Oxis(TM)) b.i.d., a 3‐month placebo‐controlled comparison with terbutaline (q.i.d.). Respiratory Medicine 1998;92(8):1040‐5. - PubMed
Faurschou 1996 {published data only}
-
- Faurschou P, Steffensen I, Jacques L. Effect of addition of inhaled salmeterol to the treatment of moderate‐to‐severe asthmatics uncontrolled on high‐dose inhaled steroids. asthmatics uncontrolled on high‐dose inhaled steroids. European Respiratory Journal 1996;9:1885‐90. - PubMed
FitzGerald 1999 {published data only (unpublished sought but not used)}
-
- FitzGerald J, Chapman K, Della Cioppa G, Stubbing D, Fairbarn M, Till M, et al. Sustained bronchoprotection, bronchodilatation, and symptom control during regular formoterol use in asthma of moderate or greater severity. Journal of Allergy & Clinical Immunology 1999;103(31):427‐35. - PubMed
Hekking 1990 {published data only}
-
- Hekking P, Maesen F, Greefhorst A, Prins J, Tan Y, Zweers P. Long term efficacy of formoterol compared with salbutamol. Lung 1990;168 Supp:76‐82. - PubMed
Hermansson1995 {published and unpublished data}
-
- Hermansson B, Jenkins R. A 4‐week comparison of salmeterol and terbutaline in adult asthma. Allergy 1995;50:551‐8. - PubMed
Juniper 1995 {published data only (unpublished sought but not used)}
-
- Juniper EF, Johnston PR, Borkhoff CM, Guyatt G, Boulet L‐P, Haukioja A. Quality of life in asthma clinical trials: a comparison of salmeterol and salbutamol. Am J Respir Crit Care Med 1995;151:66‐70. - PubMed
-
- Juniper EF, Johnston, Borkhoff C, Haukioja A. A multicentre comparison of salmeterol and salbutamol on asthma‐specific quality of life [abstract]. Quality of Life Research 1994;3:83.
Kemp 1998 {published data only}
-
- Arledge T, Liddle D, Stahl E, Rossing T. Salmeterol does not cause tolerance during long term asthma therapy. Journal of Allergy & Clinical Immunology 1996;98:1116‐9. - PubMed
-
- Kemp J, Wolfe J, Grady J, LaForce C, Stahl E, Arlidge T, et al. Salmeterol powder compared with albuterol aerosol as maintenance therapy for asthma in adolescent and adult patients. Clinical Therapeutics 1998;20(2):270‐82. - PubMed
-
- Wolfe J, LaForce C, Chervinsky P, Galant S, Lumry W, Noonan M, et al. Inhaled salmeterol powder compared with albuterol aerosol given regularly or as needed for asthma. Annals of Allergy, Asthma and Immunology 1995;74:52.
Kesten 1991 {published data only}
-
- Kesten S, Chapman KR, Broder I, et al. A three month comparison of inhaled formoterol versus four times daily inhaled albuterol in the management of stable asthma. American Review of Respiratory Disease 1991;144:622‐5. - PubMed
Kozlik‐Feldman 1996 {published data only}
-
- Kozlik‐Feldmann R, Berg A, Berdel D, Reinhardt D. Long‐term effects of formoterol and salbutamol on bronchial hyperreactivity and beta‐adrenoceptor density on lymphocytes in children with bronchial asthma. European Journal of Medical Research 1996;1:465‐70. - PubMed
Lai 1995 {published data only}
-
- Lai C, Chan C, Ho S, Hui A, Lai K. Inhaled salmeterol and albuterol in asthmatic patients receiving high‐dose inhaled corticosteroids. Chest 1995;108:36‐40. - PubMed
Leblanc 1996 {published data only (unpublished sought but not used)}
-
- Leblanc P, Knight A, Kreisman H, Borkhoff CM, Johnston PR. A placebo controlled crossover comparison of salmeterol and salbutamol in patients with asthma. American Journal of Respiratory & Critical Care Medicine 1996;154:324‐8. - PubMed
Lenney 1995 {published data only (unpublished sought but not used)}
-
- Boner A. Salmeterol: long term studies in children. European Respiratory Journal 1992;5 Suppl:318.
-
- Lenney W, Pedersen S, Boner AL, Ebbutt A, Jenkins M. Efficacy and safety of salmeterol in childhood asthma. European Journal of Pediatrics 1995;154:983‐90. - PubMed
Lipworth 1998 {published data only}
-
- Lipworth B, Tan S, Devlin M, Aiken T, Baker R, Hendrick D. Effects of treatment with formoterol on bronchoprotection against methacholine. American Journal of Medicine 1998;104(5):431‐8. - PubMed
Lundback 1993 {published data only}
Pearlman 1992 {published and unpublished data}
-
- Arledge T, Liddle D, Stahl E, Rossing T. Salmeterol does not cause tolerance during long term asthma therapy. Journal of Allergy & Clinical Immunology 1996;98:1116‐9. - PubMed
-
- D'Alonzo GE. Efficacy of inhaled salmeterol in the treatment of asthma. European Respiratory Review 1995;5:128‐32.
-
- Jenkins M, Hilton C, Kock J, Palmer J. Exacerbations of asthma in patients on salmeterol. Lancet 1991;337:913‐4. - PubMed
-
- Nathan R, Seltzer J, Kemp J, Chervinsky P, Alexander W, Liddle R, et al. Safety of salmeterol in the maintenance treatment of asthma. Annals of Allergy, Asthma, & Immunology 1995;75:243‐8. - PubMed
-
- Pearlman DS. Long‐acting beta 2‐agonist salmeterol compared with albuterol in maintenance asthma therapy. Annals of Allergy Asthma Immunology 1995;75:180‐4. - PubMed
Rutten‐van Molken 1995 {published data only (unpublished sought but not used)}
-
- Rutten‐van Molken M, Custers F, van‐Doorslater EKA, et al. Comparison of performance of four instruments in evaluating the effects of salmeterol on asthma quality of life. European Respiratory Journal 1995;8:888‐98. - PubMed
Staehr 1995 {published data only (unpublished sought but not used)}
-
- Staehr P, Vestbo I. Salmeterol improves control in asthmatic patients treated in general practice. A comparative study of salmeterol and salbutamol in patients with mild to moderate asthma. Ugeskrift for Laeger 1995;157:36‐40. - PubMed
Steffensen 1995 {published and unpublished data}
-
- Steffensen I, Faurschou P. Formoterol as inhalation powder in the treatment of patients with reversible obstructive lung diseases. A 3‐month placebo‐controlled comparison of the effects of formoterol and salbutamol, followed by a 12‐month period with formoterol alone. Ugeskrift for Laeger 1996;158:7092‐6. - PubMed
-
- Steffensen I, Faurschou P, Riska H, et al. Inhaled formoterol DP in the treatment of patients with ROAD. A 3month, placebo controlled comparison of the efficacy and safety of formoterol and salbutamol. Allergy 1995;50:657‐63. - PubMed
Taylor 1998 {published data only (unpublished sought but not used)}
Venables 1992 {published data only}
-
- Venables TA. A multicentre study in general practice to compare the efficacy and tolerability of inhaled salmeterol and terbutaline in the treatment of asthma. British Journal of Clinical Research 1992;3:125‐36.
Wenzel 1998 {published data only}
-
- Cox F, Goodwin B, Wenzel S, Rickard K, Kalberg C, Emmett A. Asthma specific quality of life in patients treated with salmeterol or albuterol. Journal of Allergy & Clinical Immunology. 1996; Vol. 97:256.
-
- Goodwin R, Cox F, Lumry W, Rickard K, Kalberg C, Emmett A. The impact of salmeterol versus albuterol on disease specific quality of life in mild to moderate asthmatics. Journal of Allergy & Clinical Immunology 1996;97(Part 3):256.
-
- Wenzel SE, Lumry W, Manning M, Kalberg C, Cox F, Emmett A, et al. Efficacy, safety, and effects on quality of life of salmeterol versus albuterol in patients with mild to moderate persistent asthma. Annals of Allergy 1998;80(6):463‐70. - PubMed
References to studies excluded from this review
Adinoff 1998 {published data only}
-
- Adinoff A, Schwartz H, Rickard K, Yancey S, Swearingen B. Salmeterol compared with current therapies in chronic asthma. Journal of Family Practice 1998;47:278‐84. - PubMed
Akpinarli 1999 {published data only}
Arvidsson1989 {published data only}
-
- Arvidsson P, Larsson S, Lofdahl CG, Melander B, Wahlander L, Svedmyr N. Formoterol, a new long acting bronchodilator for inhalation. European Respiratory Journal 1989;2:325‐30. - PubMed
Aziz 1999 {published data only}
-
- Aziz I, Lipworth BJ. A bolus of inhaled budesonide rapidly reverses airway subsensitivity and beta2‐adrenoceptor down‐regulation after regular inhaled formoterol. Chest 1999;115(3):623‐8. - PubMed
Aziz 1998 {published data only}
-
- Aziz I, Hall IP, McFarlane LC, Lipworth BJ. Beta 2 adrenoceptor regulation and bronchodilator sensitivity after regular treatment with formoterol in subjects with stable asthma. Journal of Allergy & Clinical Immunology 1998;101:337‐41. - PubMed
Aziz I Tan 1998 {published data only}
-
- Aziz I, Tan KS, Hall IP, Devlin MM, Lipworth BJ. Subsensitivity to bronchoprotection against adenosine monophosphate challenge following regular once‐daily formoterol. European Respiratory Journal 1998;12(3):580‐4. - PubMed
Baki 1998 {published data only}
-
- Baki A, Karaguzel G. Short‐term effects of budesonide, nedocromil sodium and salmeterol on bronchial hyperresponsiveness in childhood asthma. Acta Paediatrica Japonica 1998;40:247‐51. - PubMed
Becker Simons 1993 {published data only}
-
- Becker AB, Simons FER. Formoterol, a new beta2 agonist, improves chronic airway hyperresponsiveness in children with asthma. Immunology and Allergy practice 1993;14(7):18‐9.
Becker 1989 {published data only}
-
- Becker AB, Simons F. Formoterol, a new long‐acting selective beta 2‐adrenergic receptor agonist: double‐blind comparison with salbutamol and placebo in children with asthma. Journal of Allergy & Clinical Immunology 1989;84(6):891‐5. - PubMed
Bhagat 1995 {published data only}
-
- Bhagat R, Kalra S, Swystun VA, Cockcroft DW. Rapid onset of tolerance to the bronchoprotective effect of salmeterol. Chest 1995;108:1235‐9. - PubMed
Bishop 1994 {published data only}
-
- Bishop AL, Chervinsky P, Liddle R, et al. Salmeterol for treatment of reversible obstructive airways disease in children. Journal of Allergy & Clinical Immunology 1994;93:248.
Blake 1999 {published data only}
-
- Blake K, Pearlman D, Scott C, Wang Y, Stahl E, Arledge T. Prevention of exercise‐induced bronchospasm in pediatric asthma patients: a comparison of salmeterol powder with albuterol. Annals of Allergy, Asthma, & Immunology 1999;82:205‐11. - PubMed
Booth Bish 1996 {published data only}
Booth 1993 {published data only}
Boulet Cartier 1998 {published data only}
-
- Boulet L, Cartier A, Milot J, Cote J, Malo JL, Laviolette M. Tolerance to the protective effects of salmeterol on methacholine‐induced bronchoconstriction: influence of inhaled corticosteroids. European Respiratory Journal 1998;11(5):1091‐7. - PubMed
Boulet Turcot 1997 {published data only}
-
- Boulet L, Turcotte H, Boutet M, Dube J, Gagnon M, Laviolette M. Influence of salmeterol on chronic and allergen‐induced airway inflammation in mild allergic asthma : A pilot study. Current Therapeutic Research ‐ Clinical and Experimental 1997;58:240‐59.
Bowers 1997 {published data only}
-
- Bowers BW, Cox FM, Kalberg C, et al. The impact of salmeterol on asthma specific quality of life in patients reporting nocturnal asthma awakenings due to asthma [abstract]. Annals of Allergy, Asthma, & Immunology 1997;78:110.
Boyd 1995 {published data only}
-
- Boyd G. Salmeterol xinafoate in asthmatic patients under consideration for maintenance oral corticosteroid therapy. UK Study Group. European Respiratory Journal 1995;8:1494‐8. - PubMed
Brambilla 1994 {published data only}
-
- Brambilla C, Chastang C, Georges D, et al. Salmeterol compared with slow release terbutaline in nocturnal asthma: a multi center randomised double blind double dummy sequential clinical trial. Allergy 1994;49:421‐6. - PubMed
Busse 1998 {published data only}
-
- Busse WW, Casale TB, Murray JJ, Petrocella V, Cox F, Rickard K. Efficacy, safety, and impact on quality of life of salmeterol in patients with moderate persistent asthma. American Journal of Managed Care 1998;4(11):1579‐87. - PubMed
Campbell 2000 {published data only}
-
- Campbell LM, Berggren F, Emmas C. The cost effectiveness of eformoterol via Turbohaler(TM) and salmeterol via pressurised metered dose inhaler and metered dose powder inhaler in mild to moderate asthma. Journal of Medical Economics 2000;3:49‐60.
Carlsen 1995 {published data only}
-
- Carlsen K, Roksund O, Olsholt K, Nja F, Leegaard J, Bratten G. Overnight protection by inhaled salmeterol on exercise‐induced asthma in children. European Respiratory Journal 1995;8:1852‐5. - PubMed
Charpin 1992 {published data only}
-
- Charpin D, Vervloet D. Quality of life in moderate chronic asthma: salmeterol versus current treatments [abstract]. Allergy 1992;47(12 Suppl):230.
Chervinsky 1999 {published data only}
-
- Chervinsky P, Goldberg P, Galant S, Wang Y, Arledge T, Welch MB, et al. Long‐term cardiovascular safety of salmeterol powder pharmacotherapy in adolescent and adult patients with chronic persistent asthma: A randomized clinical trial. Chest 1999;115(3):642‐8. - PubMed
Cheung 1992 {published data only}
-
- Cheung D, Timmers MC, Zwinderman AH, et al. Long term effects of a long acting beta adrenoceptor agonist in patients with mild asthma. New England Journal of Medicine 1992;327:1198‐203. - PubMed
Clark 1993 {published data only}
-
- Clark CE, Ferguson AD, Siddorn J. Respiratory arrests in young asthmatics on salmeterol. Respiratory Medicine 993;87:227‐8. - PubMed
Clauzel 1998 {published data only}
-
- Clauzel AM, Molimard M, Gros V, Lepere E, Febvre N, Michel FB. Use of formoterol dry powder administered for three months via a single dose inhaler in 1,380 asthmatic patients. Journal of Investigational Allergology and Clinical Immunology 1998;8:265‐0. - PubMed
Cockcroft 1997 {published data only}
-
- Cockcroft DW, Swystun VA, Bhagat R. Salmeterol and airway response to allergen. Canadian Respiratory Journal 1997;4:37‐40.
Dahl 1991 {published data only}
-
- Dahl R, Earnshaw JS, Palmer JBD. Salmeterol: a 4 week study of a long acting beta2 adrenoceptor agonist for the treament of reversible airways disease. European Respiratory Journal 1991;4:1178‐84. - PubMed
Derom 1992 {published data only}
-
- Derom EY, Pauwels RA, Straten MEF. The effect of inhaled salmeterol on methacholine responsiveness in subjects with asthma up to 12 hours. Journal of Allergy & Clinical Immunology 1992;89:811‐5. - PubMed
Di‐Lorenzo 1995 {published data only}
-
- Di‐Lorenzo G, Morici G, Norrito F, Mansueto P, Melluso M, Purello‐D'Ambrosio F, et al. Comparison of the effects of salmeterol and salbutamol on clinical activity and eosinophil cationic protein serum levels during the pollen season in atopic asthmatics. Clinical and experimental allergy 1995;25(10):951‐6. - PubMed
Droszcz 1999 {published data only}
-
- Droszcz P, Droszcz W. Results of 30 days and 12 months treatment with salmeterol in patients with asthma and COPD. Polske Merkuriusz Lekarski 1999;6:266‐7. - PubMed
Faurschou 1991 {published data only}
-
- Faurschou P. Chronic dose‐ranging studies with salmeterol. European Respiratory Journal 1991;1(4):282‐87.
Faurschou 1992 {published data only}
-
- Faurschou P. Salmeterol and Salbutamol: long term efficacy and safety. European Respiratory Journal 1992;5 Supp (15):317‐8.
Faurschou Dahl 1997 {published data only}
-
- Faurschou P, Dahl R, Jeffery P, et al. Comparison of the anti‐inflammatory effects of fluticasone and salmeterol in asthma: A placebo controlled, DB CO with bronchoscopy, bronchial methacholine provocation and lavage. European Respiratory Journal 1997;10 Suppl:243.
Fitzpatrick 1990 {published data only}
Fuglsang 1998 {published data only}
-
- Fuglsang G, Vikre‐Jorgensen J, Agertoft L, Pedersen S. Effect of salmeterol treatment on nitric oxide level in exhaled air and dose‐response to terbutaline in children with mild asthma. Pediatric Pulmonology 1998;25(5):314‐21. - PubMed
Gardiner 1994 {published data only}
-
- Gardiner PV, Ward C, Booth H, et al. Effect of eight weeks treatment with salmeterol on bronchoalveolar lavage inflammatory indices in asthmatics. American Journal of Respiratory & Critical Care Medicine 1994;150:1006‐11. - PubMed
Giannini 1995 {published data only}
-
- Giannini D, Carletti A, Dente FL, et al. Tolerance to salbutamol in allergen induced bronchoconstriction. American Journal of Respiratory & Critical Care Medicine 1995;151:A39.
Giannini Bacci 1999 {published data only}
-
- Giannini D, Bacci E, Dente FL, Franco A, Vagagginin B, Testi R, et al. Inhaled beclomethasone diproprionate reverts tolerance to the protective effect of salmeterol on allergen challenge. Chest 1999;115:629‐34. - PubMed
Gong 1996 {published data only}
-
- Gong H, Linn WS, Shamou DA, et al. Effect of inhaled salmeterol on sulfur dioxide induced bronchoconstriction in asthmatic subjects. Chest 1996;110:1229‐35. - PubMed
Gotz 1995 {published data only}
-
- Gotz MH, Taak NK. The efficacy and safety of inhaled salmeterol 50mcg bd compared with salbutamol 200mcg prn in children with asthma [abstract]. European Respiratory Journal 1995;8 Suppl (19):517.
Green 1992 {published data only}
Greening 1994 {published data only}
-
- Greening AP, Ind PW, Norhtfield M, et al. Added salmeterol versus higher dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Lancet 1994;344:219‐24. - PubMed
Grove 1995 {published data only}
-
- Grove A, Lipworth BJ. Bronchodilator subsensitivity to salbutamol after twice daily salmeterol in asthmatic patients. Lancet 1995;346:201‐6. - PubMed
Grove Lipworth 1996 {published data only}
Hacki 1993 {published data only}
-
- Hacki M, Ritter A, Medici T. Formoterol: three years therapy in asthma. European Respiratory Journal 1993;6 Suppl (17):529.
Hyland 1994 {published data only}
-
- Hyland ME, Kenyon CAP, Jacobs P. Sensitivity of quality of life domains and constructs to longitudinal change in a clinical trial comparing salmeterol and placebo in asthmatics. Quality of Life Research 1994;3:121‐6. - PubMed
Jartti 1998 {published data only}
-
- Jartti TT, Kaila TJ, Tahvanainen KUO, Kuusela TA, Vanto TT, Valimaki IAT. Altered cardiovascular autonomic regulation after salmeterol treatment in asthmatic children. Clinical Physiology 1998;18(4):345‐53. - PubMed
Jones 1994 {published data only}
Juniper 1999 {published data only}
-
- Juniper E, Svensson K, O'Byrne P, Barnes P, Bauer C, Lofdahl C‐G, et al. Asthma quality of life during 1 year of treatment with budesonide with or without formoterol. European Respiratory Journal 1999;14(5):1038‐43. - PubMed
Kalra 1996 {published data only}
-
- Kalra S, Swystun V, Bhagat R, Cockcroft DW. Inhaled corticosteroids do not prevent the development of tolerance to the bronchoprotective effect of salmeterol. Chest 1996;109:953‐6. - PubMed
Kavuru 2000 {published data only}
-
- Kavuru M, Melamed J, Gross G, LaForce C, House K, Prillaman B, et al. Salmeterol and fluticasone propionate combined in a new powder inhalation device for the treatment of asthma: A randomized, double‐blind, placebo‐controlled trial. Journal of Allergy & Clinical Immunology 2000;105(6):1108‐16. - PubMed
Kemp Cook 1998 {published data only}
-
- Kemp J, Cook D, Incaudo G, Corren J, Kalberg C, Emmett A, et al. Salmeterol improves quality of life in patients with asthma requiring inhaled corticosteroids. Salmeterol Quality of Life Study Group. Journal of Allergy & Clinical Immunology 1998;101:188‐95. - PubMed
Kemp 1993 {published data only}
-
- Kemp J, Bukstein D, Busse W. Effective salmeterol, salbutamol and placebo in the prevention of exercise induced bronchospasm. Chest 1993;104 Suppl:10.
Kemp DeGraff 1999 {published data only}
-
- Kemp J, DeGraff A, Pearlman D, Chervinsky P, Galant S, Goldberg P, et al. A 1‐year study of salmeterol powder on pulmonary function and hyperresponsiveness to methacholine. Journal of Allergy & Clinical Immunology 1999;104(6):1189‐97. - PubMed
Kemp Dockhorn 1994 {published data only}
-
- Kemp JP, Dockhorn RJ, Busse WW, et al. Prolonged effect of inhaled salmeterol against exercise induced bronchoconstriction after chronic dosing with salmeterol. American Journal of Respiratory & Critical Care Medicine 1994;150:1612‐5. - PubMed
Kesten 1992 {published data only}
-
- Kesten S, Chapman KR, Broder I, Cartier A, Hyland RH, Knight A, et al. Sustained improvement in asthma with long‐term use of formoterol fumarate. Annals of Allergy 1992;69(5):415‐20. - PubMed
Kia 1997 {published data only}
-
- Kia Soong T, Grove A, McLean A, Gnosspelius Y, Hall IP, Lipworth BJ. Systemic corticosteroid rapidly reverses bronchodilator subsensitivity induced by formoterol in asthmatic patients. American Journal of Respiratory & Critical Care Medicine 1997;156(1):28‐35. - PubMed
Kraft 1997 {published data only}
-
- Kraft M, Wenzel SE, Bettinger CM, Martin RJ. The effect of salmeterol on nocturnal symptoms, airway function and inflammation in asthma. Chest 1997;111:1249‐54. - PubMed
Langley 1998 {published data only}
-
- Langley SJ, Masterson CM, Batty EP, Woodcock A. Bronchodilator response to salbutamol after chronic dosing with salmeterol or placebo. European Respiratory Journal 1998;11:1081‐5. - PubMed
Langton 1995 {published data only}
-
- Langton Hewer S, Hobbs J, French D, Lenney W. Pilgrim's progress: the effect of salmeterol in older children with chronic severe asthma. Respiratory Medicine 1995;89:435‐40. - PubMed
Li Ward 1999 {published data only}
-
- Li X, Ward C, Thien F, Bish R, Bamford T, Bao X, et al. An antiinflammatory effect of salmeterol, a long‐acting beta2 agonist, assessed in airway biopsies and bronchoalveolar lavage in asthma. American Journal of Respiratory & Critical Care Medicine 1999;160:1493‐9. - PubMed
Li 1998 {published data only}
-
- Li X, Bamford T, Ward C, et al. Influence of salmeterol on eosinophil inflammation in bronchial biopsies from asthmatics on inhaled steroid. European Respiratory Journal 1998;12 Suppl:157.
Lipworth Aziz 2000 {published data only}
-
- Lipworth B, Aziz I. Bronchodilator response to albuterol after regular formoterol and effects of acute corticosteroid administration. Chest 2000;117(1):156‐62. - PubMed
Lipworth Demps 2000 {published data only}
-
- Lipworth BJ, Dempsey OJ, Aziz I. Functional antagonism with formoterol and salmeterol in asthmatic patients expressing the homozygous Glycine‐16 beta2 adrenoceptor polymorphism. Chest 2000;118(2):321‐8. - PubMed
Lipworth Aziz 1999 {published data only}
-
- Lipworth BJ, Aziz I. A high dose of albuterol does not overcome bronchoprotective susbsensitivity in asthmatic subjects deceiving regular salmeterol or formoterol. Journal of Allergy & Clinical Immunology 1999;103:88‐92. - PubMed
Lockey 1999 {published data only}
-
- Lockey R, DuBuske L, Friedman B, Petrocella V, Cox F, Rickard K. Nocturnal Asthma. Effect of Salmeterol on Quality of Life and Clinical Outcomes. Chest 1999;115:666‐73. - PubMed
Lotvall 1992 {published data only}
-
- Lotvall J, Lunde H, Ullman A, Tornquist H, Svedmyr N. Twelve months, treatment with inhaled salmeterol in asthmatic patients. effects on b2receptor function and inflammatory cells. Allergy 1992;47:477‐83. - PubMed
Mahajan 1998 {published data only}
-
- Mahajan P, Stahl E, Arledge T. Quality of life in pediatric asthma patients treated with salmeterol and impact on the daily activities of their parents. Pediatric Asthma Allergy & Immunology 1998;12(1):21‐8.
Malo 1992 {published data only}
-
- Malo J‐L, Ghezzo H, Trudeau C. Salmeterol a new inhaled beta2 agonist, has a longer blocking effect than albuterol on hyperventilation induced bronchoconstriction. Journal of Allergy & Clinical Immunology 1992;89:567‐74. - PubMed
Malozowski 1998 {published data only}
-
- Malozowski S, Stadel BV, Pian LP, Simons FER. Comparison of beclomethasone, salmeterol, and placebo in children with asthma. New England Journal of Medicine 1998;339(10):704‐5. - PubMed
Mann 1996 {published data only}
-
- Mann RD, Kubota K, Pearce G, et al. Salmeterol: a study by prescription event monitoring in a UK cohort of 15,407 patients. Journal of Clinical Epidemiology 1996;49:247‐50. - PubMed
Martin 1999 {published data only}
-
- Martin R, Kraft M, Beaucher Wea. Comparative study of extended release albuterol sulfate and long‐acting inhaled salmeterol xinafoate in the treatment of nocturnal asthma. Annals of Allergy, Asthma, & Immunology 1999;83(2):121‐6. - PubMed
McIvor 1998 {published data only}
-
- McIvor RA, Pizzichini E, Turner MO, et al. Potential masking effects of salmeterol on airway inflammation in asthma. American Journal of Respiratory & Critical Care Medicine 1998;158:924‐30. - PubMed
Meijer 1995 {published data only}
-
- Meijer GG, Postma DS, Mulder PGH, et al. Long term circadian effects of salmeterol in asthmatic children treated with inhaled corticosteroids. American Journal of Respiratory & Critical Care Medicine 1995;152:1887‐92. - PubMed
Midgren 1992 {published data only}
-
- Midgren B, Melander B, Persson G. Formoterol, a new long acting beta2 agonist, inhaled twice daily in stable asthmatic subjects. Chest 1992;101:1019‐22. - PubMed
Molimard 2001 {published data only}
-
- Molimard M, Bourcereau V, LeGros V, Bourdeix I, Leynadier F, Duroux P. Comparison between formoterol 12mcg BID and on‐demand salbutamol in moderate persistent asthma. Respiratory Medicine 2001;95:65‐70. - PubMed
Nathan 1999 {published data only}
-
- Nathan R, Pinnas J, Schwartz H, Grossman J, Yancey S, Emmett A, et al. A six‐month, placebo‐controlled comparison of the safety and efficacy of salmeterol or beclomethasone for persistent asthma. Annals of Allergy 1999;82(6):521‐9. - PubMed
Nelson 1999 {published data only}
-
- Nelson H, Berkowitz R, Tinkelman D, Emmett A, Rickard K, Yancey S. Lack of Subsensitivity to Albuterol After Treatment with Salmeterol in Patients with Asthma. American Journal of Respiratory & Critical Care Medicine 1999;159:1556‐61. - PubMed
Nelson 1998 {published data only}
-
- Nelson J, Strauss L, Skowronski M, Ciufo R, Novak R, McFadden EJ. Effect of long‐term salmeterol treatment on exercise‐induced asthma. New England Journal of Medicine 1998;339(141‐6). - PubMed
Newnham 1993 {published data only}
-
- Newnham DM, Ingram CG, Earnshaw J, et al. Salmeterol provides prolonged protection against exercise induced bronchoconstriction in a majority of subjects with mild stable asthma. Respiratory Medicine 1993;87:439‐44. - PubMed
Newnham 1994 {published data only}
-
- Newnham DM, McDevitt DG, Lipworth BJ. Bronchodilator subsensitivity after chronic dosing with eformoterol in patients with asthma. American Journal of Medicine 1994;97(1):29‐37. - PubMed
Newnham 1995 {published data only}
Nichol 1990 {published data only}
-
- Nichol G, Nix A, Barnes P, Chung K. Prolonged attenuation of BHR to methacholine by long acting b2adrenoceptor agonist formoterol. American Review of Respiratory Disease 1990;141:A 210.
Nielsen 1999 {published data only}
-
- Nielsen L, Pedersen B, Faurschou P, Madsen F, Wilcke J, Dahl R. Salmeterol reduces the need for inhaled corticosteroid in steroid‐dependent asthmatics. Respiratory Medicine 1999;93(12):863‐8. - PubMed
Nix 1990 {published data only}
Norhaya 1999 {published data only}
-
- Norhaya M, Yap T, Zainudin B. Addition of inhaled salmeterol to inhaled corticosteroids in patients with poorly controlled nocturnal asthma. Respirology 1999;4(1):77‐81. - PubMed
Nowak 1992 {published data only}
-
- Nowak D, Jorres R, Rabe KF, et al. Salmeterol protects agonist hyperventilation induced bronchoconstriction over 12 hours. European Journal of Clinical Pharmacology 1992;43:591‐5. - PubMed
Palmer 1992 {published data only}
-
- Palmer JBD, Stuart AM, Shepherd GL, Viskum K. Inhaled salmeterol in the treatment of patients with moderate to severe ROAD‐ a 3 mth comparison of the efficacy and safety of bd salmeterol (100mcg) and salmeterol (50mcg). Respiratory Medicine 1992;86:409‐17. - PubMed
Pauwels 1997 {published data only}
-
- Pauwels RA, Lofdahl CG, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. New England Journal of Medicine 1997;337:1405‐11. - PubMed
Pearlman 1996 {published data only}
-
- Pearlman DS, Bronsky E, Chervinsky P, et al. Inhaled salmeterol powder compared with placebo administered over 12 weeks to children with mild to moderate asthma. American Journal of Respiratory & Critical Care Medicine 1996;153(4):A76.
Pearlman 1999 {published data only}
-
- Pearlman D, Stricker W, Weinstein S, Gross G, Chervinsky P, Woodring A, et al. Inhaled salmeterol and fluticasone: A study comparing monotherapy and combination therapy in asthma. Annals of Allergy 1999;82(3):257‐65. - PubMed
Pederson 1993 {published data only}
-
- Pederson B, Dahl R, Larsen BB, et al. The effect of salmeterol on the early and late phase reaction to bronchial allergen and post challenge variation in BHR, blood eosinophils, serum ECP and serum eosinophil protein X. Allergy 1993;48:377‐82. - PubMed
Quebe‐Fehling 1996 {published data only}
-
- Quebe‐Fehling E, Brambilla R, Bromly C L, Fishwick K, Walters E H, Hendrick DJ. The duration of action of inhaled formoterol dry powder. British Journal of Clinical Practice 1996;50(8):446‐9. - PubMed
Rabe 1993 {published data only}
-
- Rabe KF, Jorres R, Nowak D, et al. Comparison of the effects of salmeterol and formoterol on airway tone and responsiveness over 24 hours in bronchial asthma. American Review of Respiratory Disease 1993;147:1436‐41. - PubMed
Ramage 1994 {published data only}
-
- Ramage L, Cree IA, Dhillon DP. Comparison of salmeterol with placebo in mild asthma: effect on peripheral blood phagocyte function and cytokine levels. International Archives of Allergy & Immunology 1994;105:181‐4. - PubMed
Ramage Lipworth 1994 {published data only}
-
- Ramage L, Lipworth BJ, Ingram CG, et al. Reduced protection against exercise induced bronchoconstriction after chronic dosing with salmeterol. Respir Med 1994;88:363‐8. - PubMed
Ramsdale 1991 {published data only}
-
- Ramsdale E, Otis J, Kline P, Gontovnick L, Hargreave F, O'Byrne P. Prolonged protection against methacholine induced bronchoconstriction by the inhaled b2 agonist formoterol. American Review of Respiratory Disease 1991;143:998‐1001. - PubMed
Ringbaek 1996 {published data only}
-
- Ringbaek TJ, Soes‐Petersen U, Christensen M, Iversen ET, Rasmussen FV. Salmeterol compared with salbutamol controlled release in patients with moderate bronchial asthma. Ugeskrift for Laeger 1996;158:3940‐3. - PubMed
Roberts 1999 {published data only}
-
- Roberts J, Bradding P, Britten K, Walls A, Wilson S, Gratziou C, et al. The long‐acting beta2‐agonist salmeterol xinafoate: effects on airway inflammation in asthma. European Respiratory Journal 1999;14(2):275‐82. - PubMed
Roberts 1992 {published data only}
-
- Roberts B, Bradding P, Holgate S. Effects of a six week course of salmeterol on bronchial reactivity. Thorax 1992;47:230p.
Rosenthal 1997 {published data only}
-
- Rosenthal R, Chervinsky P, DeGraaf A, et al. Long term regular treatment with salmeterol is effective in protecting against bronchial hyperresponsiveness as measured by methacholine challenge in asthmatics [abstract]. Journal of Allergy & Clinical Immunology 1997;99 Suppl:323.
Rosenthal 1999 {published data only}
-
- Rosenthal R, Busse W, Kemp J, Baker J, Kalberg C, Emmett A, et al. Effect of long‐term salmeterol therapy compared with as‐needed albuterol use on airway hyperresponsiveness. Chest 1999;116(3):595‐602. - PubMed
Russell 1995 {published data only}
-
- Russell G, Williams DAJ, Weller P, Price JF. Salmeterol xinafoate in children on high dose inhaled steroids. Annals of Allergy, Asthma and Immunology 1995;75(5):423‐8. - PubMed
Schaaning 1996 {published data only}
-
- Schaaning J, Vilsvik J, Henriksen AH. Efficacy and duration of salmeterol in protecting against exercise induced bronchoconstriction. Annals of Allergy, Asthma, & Immunology 1996;76:57‐60. - PubMed
Schreurs 1996 {published data only}
-
- Schreurs A, Sinninghe Damste H, Graaff C, Greefhorst A. A dose‐response study with formoterol Turbuhaler as maintenance therapy in asthmatic patients. European Respiratory Journal 1996;9(8):1678‐83. - PubMed
Self 1998 {published data only}
-
- Self T, Rumbak MJ, Kelso T, Eberle L, Abou‐Shala N, Learned CC, et al. Does salmeterol facilitate 'step‐down' therapy in patients with asthma receiving moderate to high doses of inhaled corticosteroids?. Current Therapeutic Research, Clinical & Experimental 1998;59:803‐11.
Shapiro 2000 {published data only}
-
- Shapiro G, Lumry W, Wolfe J, Given J, White M, Woodring A, et al. Combined salmeterol 50 mug and fluticasone propionate 250 in the diskus device for the treatment of asthma. American Journal of Respiratory & Critical Care Medicine 2000;161:527‐34. - PubMed
Shepherd 1991 {published data only}
-
- Shepherd G, Jenkins W, Alexander J. Asthma exacerbations in patients taking regular salmeterol, or salbutamol for symptoms. Lancet 1991;337(8754):1424. - PubMed
Sichletidis 1993 {published data only}
-
- Sichletidis L, Daskalopoulou E, Kyriakis G, et al. Comparative efficacy of salbutamol and salmeterol in exercise induced asthma. Journal of Internal Medicine Research 1993;21:81‐8. - PubMed
Siergiejko 1998 {published data only}
-
- Siergiejko Z, Zietkowsk1 Z, Rogalewska A, Chyrek‐Borowska S. Clinical evaluation of 12 week salmeterol therapy in allergic asthma patients. Pneumonologia i Alergololia Polski 1998;66:450‐5. - PubMed
Simons 1992 {published data only}
-
- Simons F, Soni N, Watson W, Becker A. Bronchodilator and bronchoprotective effects of salmeterol in young patients with asthma. Journal of Allergy & Clinical Immunology 1992;90:840‐6. - PubMed
Simons 1997 {published data only}
-
- Simons FER, et al. A one year placebo controlled study of the efficacy and safety of beclomethasone DP versus salmeterol in glucocorticoid naive children with asthma. Journal of Allergy & Clinical Immunology 1997;99 Suppl:402.
Simons 2 1997 {published data only}
-
- Simons FER. A comparison of Beclomethasone, salmeterol and placebo in children with asthma. New England Journal of Medicine 1997;337(23):1659‐65. - PubMed
Simons Gerst 1997 {published data only}
-
- Simons FER, Gerstner TV, Cheang MS. Tolerance to the bronchoprotective effect of salmeterol in adolescents with exercise‐induced asthma using concurrent inhaled glucocorticoid treatment. Pediatrics 1997;99(5):655‐9. - PubMed
Smyth 1993 {published data only}
Starke 1996 {published data only}
-
- Starke I, Luce P. The efficacy and safety of inhaled salmeterol 50 microg bd in older patients with reversible airflow obstruction. Age & Ageing 1996;25:67‐71. - PubMed
Sussman 1995 {published data only}
-
- Sussman HS. Continuous eformoterol and beta 2‐receptor responsiveness.. British Journal of Clinical Practice 1995;81(Symposium Supplement.):16‐7. - PubMed
Tan 1997 {published data only}
-
- Tan KS, Grove A, McLean A, Gnosspelius Y, Hall IP, Lipworth BJ. Systemic corticosteroid rapidly reverses bronchodilator subsensitivity induced by formoterol in asthmatic patients. American Journal of Respiratory & Critical Care Medicine 1997;156:28‐35. - PubMed
Tattersfield 2001 {published data only}
-
- Tattersfield A, Lofdahl C‐G, Postma D, Eivindson A, Schreurs A, Rasidikis A, et al. Comparison of formoterol and terbutaline for as‐needed treatment of asthma: as randomised trial. The Lancet 2001;357:257‐61. - PubMed
Tattersfield 1999 {published data only}
-
- Tattersfield A, Postma D, Barnes P, Svensson K, Bauer C, O'Byrne P, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations. The FACET International Study Group. American Journal of Respiratory & Critical Care Medicine 1999;160(2):594‐9. - PubMed
Taylor 1992 {published data only}
-
- Taylor IK, O'Shaugnessy KM, Choudry NB, et al. A comparative study in atopic subjects with asthma of the effects of salmeterol and salbutamol on allergen induced bronchoconstriction, increase in AR ,increase in urinary leukotriene E4 excretion. Journal of Allergy & Clinical Immunology 1992;89:575‐83. - PubMed
Totterman 1998 {published data only}
-
- Totterman KJ, Huhti L, Sutinen E, Backman R, Pietinalho A, Falck M, et al. Tolerability to high doses of formoterol and terbutaline via Turbuhaler(TM) for 3 days in stable asthmatic patients. European Respiratory Journal 1998;12(3):573‐9. - PubMed
Turner 1998 {published data only}
-
- Turner M, Johnston P, Pizzichini E, et al. Anti‐inflammatory effects of salmeterol compared with beclomethasone in eosinophilic mild exacerbations of asthma: a randomized, placebo controlled trial. Canadian Respiratory Journal 1998;5:261‐8. - PubMed
Twentyman 1990 {published data only}
-
- Twentyman OP, Finnerty JP, Harris A, et al. Protection against allergen induced asthma by salmeterol. Lancet 1990;336:1338‐42. - PubMed
Ullman 1988 {published data only}
Ullman 1990 {published data only}
-
- Ullman A, Hedner J, Svedmyr N. Inhaled salmeterol and salbutamol in asthmatic patients. An evaluation of asthma symptoms and the possible development of tachyphylaxis. American Review of Respiratory Disease 1990;142:571‐5. - PubMed
Van der Molen 1996 {published data only}
Van der Molen 1998 {published data only}
-
- Molen T, Sears MR, Graaff CS, Postma DS, Meyboom‐de Jong B. Quality of life during formoterol treatment: comparison between asthma‐specific and generic questionnaires. European Respiratory Journal 1998;12(1):30‐4. - PubMed
Verberne 1991 {published data only}
-
- Verberne A, Lenney W, Kerribyjn K. A 3 way crossover study comparing twice daily dosing of salmeterol 25mcg and 50mcg with placebo in children with mild to moderate reversible airways disease[abstract]. American Review of Respiratory Disease 1991;143(suppl 2):20.
Verberne 1996 {published data only}
-
- Verberne AAPH, Hop WCJ, Creyghton FBM, et al. Airway responsiveness after a single dose of salmeterol and during four months of treatment in children with asthma. Journal of Allergy & Clinical Immunology 1996;97:938‐46. - PubMed
Verberne 1997 {published data only}
-
- Verberne A, Frost C, Roorda R, Laag H, Kerrebijn K. One year treatment with salmeterol compared with beclomethasone in children with asthma. The Dutch Paediatric Asthma Study Group. American Journal of Respiratory & Critical Care Medicine 1997;156:688‐95. - PubMed
Verberne 1998 {published data only}
-
- Verberne AA, Frost C, Duiverman EJea. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. The Dutch Asthma Study Group. American Journal of Respiratory & Critical Care Medicine 1998;158:213‐9. - PubMed
Verberne 2000 {published data only}
-
- Verberne A, Jongste J. Long‐acting beta2‐agonists in childhood asthma: Don't change a winning team (yet). Pediatric Pulmonology 2000;29(3):169‐171. - PubMed
Verini 1998 {published data only}
-
- Verini M, Verrotti A, Greco R, Chiarelli F. Comparison of the bronchodilator effect of inhaled short‐ and long‐acting beta2‐agonists in children with bronchial asthma. A randomised trial. Clinical Drug Investigation 1998;16(1):19‐24. - PubMed
Viskum 1990 {published data only}
-
- Viskum K. Inhaled salmeterol improves control in moderate to severe asthmatics: a 3mth study. European Respiratory Journal 1990;3 Suppl (10):235.
Von Berg 1996 {published data only}
-
- Berg A, Blic J, Rosa M, et al. Regular salbutamol xinafoate compared with salbutamol as required in children with moderate asthma [abstract]. American Journal of Respiratory & Critical Care Medicine 1996;4:A76.
Von Berg 1998 {published data only}
-
- Berg A, Blic J, Rosa M, et al. A comparison of regular salmeterol versus as required salbutamol in asthmatic children. Respiratory Medicine 1998;92:292‐9. - PubMed
Wallaert 1999 {published data only}
-
- Wallaert B, Brun P, Ostinelli J, Murciano D, Champel F, Blaive B, et al. A comparison of two long acting beta agonists, oral bambuterol and inhaled salmeterol, in the treatment of asthmatics with nocturnal symptoms. Respiratory Medicine 1999;93:33‐8. - PubMed
Wallin 1999 {published data only}
Wallin 1990 {published data only}
-
- Rosenhall L, Sandstrom T, Wallin A. Formoterol, a long acting inhaled beta‐2 agonist, twice daily for 1 year in asthmatic patients. American Review of Respiratory Disease 1990;141:A210.
Walters 1992 {published data only}
-
- Walters H. Quality of life in long term studies. European Respiratory Journal 1992;5 Suppl:318.
Weinstein 1997 {published data only}
-
- Weinstein S, Chervinsky P, Pollard SJ, Bronsky EA, Nathan RA, Prenner B, et al. A one‐week dose‐ranging study of inhaled salmeterol in children with asthma. Journal of Asthma 1997;34(1):43‐52. - PubMed
Weinstein 1998 {published data only}
-
- Weinstein S, Pearlman D, Bronsky E, Byrne A, Arledge T, Liddle R, et al. Efficacy of salmeterol xinafoate powder in children with chronic persistent asthma. Annals of Allergy, Asthma, & Immunology 1998;81:51‐8. - PubMed
Wilding 1997 {published data only}
Wolfe 2000 {published data only}
-
- Wolfe J, Kreitzer S, Chervinsky P, Lawrence M, Wang Y, Reilly D, et al. Comparison of powder and aerosol formulations of salmeterol in the treatment of asthma. Annals of Allergy 2000;84(3):334‐40. - PubMed
Wong 1997 {published data only}
-
- Wong AG, O'Shaughnessy AD, Walker CM, Sears MR. Effects of long‐acting and short‐acting beta‐agonists on methacholine dose‐response curves in asthmatics. European Respiratory Journal 1997;10:330‐6. - PubMed
Woolcock 1996 {published data only}
-
- Woolcock A, Lundback B, Ringdal N, et al. Comparison of the addition of salmeterol to inhaled steroids with the doubling of the dose of inhaled steroids. American Journal of Respiratory & Critical Care Medicine 1996;153:1481‐8. - PubMed
Wronska 1998 {published data only}
-
- Wronska J, Chazan R, Mazurek J, Droszcz W. Treatment with salmeterol and quality of life in patients with asthma. Pneumonologia i Alergologi Polski 1998;66:193‐7. - PubMed
Yates 1995 {published data only}
-
- Yates DH, Sussman HS, Shaw MJ, Barnes PJ, Chung KF. Regular formoterol treatment in mild asthma. Effect on bronchial responsiveness during and after treatment. American Journal of Respiratory & Critical Care Medicine 1995;152:1170‐4. - PubMed
Yates 1997 {published data only}
-
- Yates DH, Worsdell M, Barnes PJ. Effect of regular salmeterol treatment on albuterol‐induced bronchoprotection in mild asthma. American Journal of Respiratory & Critical Care Medicine 1997;156(3):988‐91. - PubMed
Zarkovic 1998 {published data only}
-
- Zarkovic J, Gotz MH, Holgate ST, Taak NK. Effect of long‐term regular salmeterol treatment in children with moderate asthma. Clinical Drug Investigation 1998;15(3):169‐75.
Zellweger 1994 {published data only}
-
- Zellweger JP, Plenninger M, Ruff P. 24 hour protective effect of salmeterol 50mg v formoterol 24 mg against methacholine induced bronchoconstriction in mild to moderate asthmatic patients: a randomised double blind crossover single dose trial. European Respiratory Journal 1994 Suppl (18);suppl 18:422.
References to studies awaiting assessment
Cloosterman 2001 {published data only}
-
- Cloosterman SG, Bijl‐Hofland ID, Herwaarden CL, et al. A placebo‐controlled clinical trial of regular monotherapy with short‐acting and long‐acting beta(2)‐agonists in allergic asthmatic patients. Chest 2001;119:1306‐15. - PubMed
Rhee 1997 {published data only}
-
- Rhee YK. A comparison of salmeterol with salbutamol inhalation in treatment of mild to moderate asthma. Tuberculosis and Respiratory Diseases 1997;44(4):815‐21.
Sprogoe‐Jakobsen 92 {published data only}
-
- Sprogoe‐Jakobsen U, Viktrup L, Davidsen O, Viskum K. Bronchial asthma treated with long‐acting beta 2 agonist. Comparison between formoterol (12 mu/g) inhaled twice daily and salbutamol (200 mu/g) inhaled 4 times daily. Ugeskr‐Laeger 1992;154(3):325‐8. - PubMed
Stalenheim 1994 {published data only}
-
- Stalenheim G, Wegener T, Grettve L, et al. Efficacy and tolerance of a 12‐week treatment with inhaled formoterol in patients with reversible obstructive lung disease. Respiration 1994;61:305‐9. - PubMed
Thomson 1998 {published data only}
-
- Thomson N, Angus R, Quebe‐Fehling E, Brambilla R. Efficacy and tolerability of formoterol in elderly patients with reversible obstructive airways disease. Respiratory Medicine 1992;92:562‐7. - PubMed
Additional references
AAACI 1993
-
- American Academy of Allergy and Clinical Immunology, Executive committee. Inhaled beta2 agonist in asthma. Journal of Allergy & Clinical Immunology 1993;91:1234‐7. - PubMed
Abramson 2001
-
- Abramson M, Bailey M, Couper F, et al. Are asthma medications and management related to deaths from asthma?. American Journal of Respiratory & Critical Care Medicine 2001;163:12‐8. - PubMed
Adcock 1996
-
- Adcock I, Stevens D, Barnes P. Interactions of glucocorticoids and beta2 agonists. European Respiratory Journal 1996;9:160‐9. - PubMed
Adkins 1997
-
- Adkins JC, McTavish D. Salmeterol. A review of its pharmacological properties and clinical efficacy in the management of children with asthma. Drugs 1997;54(2):331‐54. - PubMed
Bartow 1998
-
- Bartow RA, Brogen RN. Formoterol. An update of its pharmacological properties and therapeutic efficacy in the management of asthma. Drugs 1998;55:303‐22. - PubMed
Beasley 1999
-
- Beasley R, Pearce N, Crane J, Burgess C. Beta‐agonists: What is the evidence that their use increases the risk of asthma morbidity and mortality?. Journal of Allergy & Clinical Immunology 1999;104 Suppl (2 ll):18‐30. - PubMed
Bisgard 2000
-
- Bisgaard H. Long‐acting beta(2)‐agonists in management of childhood asthma: A critical review of the literature. Pediatric Pulmonology 2000;29(3):221‐34. - PubMed
Boulet 1994
-
- Boulet LP. Long versus short acting beta agonists. Drugs 1994;47:207‐22. - PubMed
Bousquet 2000
-
- Bousquet J. Global initiative for asthma (GINA) and its objectives. Clinical & Experimental Allergy 2000;30 Supp (1):2‐5. - PubMed
BTS 1993
-
- British Thoracic Society, British Paediatric Association, et al. Guidelines on the management of asthma. Thorax 1993;48 Suppl:1‐24.
BTS 1997
-
- British Thoracic Society, NAtional Asthma Caompaign, Royal College of Pysicians, et al. The British guidelines on Asthma Management 1995 review and position statement. Thorax 1997;52 Suppl:1.
Campbell 1976
-
- Campbell A. Mortality from asthma and bronchodilator aerosols. Medical Journal of Australia 1976;1:386‐91. - PubMed
Campbell 1999
-
- Campbell LM, Anderson TJ, Parashchak MR, Burke CM, Watson AS, Turbitt ML. [A comparison of the efficacy of long‐acting beta 2‐agonists: eformoterol via Turbohaler and salmeterol via pressurized metered dose inhaler or Accuhaler, in mild to moderate asthmatics. Respiratory Medicine]. Respiratory Medicine 1999;93(7):236‐44. - PubMed
Cockcroft 1993
-
- Cockcroft DW, McParland CP, Britto SA. Regular inhaled salbutamol and airway responsiveness to allergen. Lancet 1993;342:833‐7. - PubMed
Cockcroft 1996
Crane 1989
-
- Crane J, Pearce N, Flatt A, et al. Prescribed fenoterol and death from asthma in New Zealand 1981‐1983. A case control study. Lancet 1989;1:917‐22. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7(3):177‐88. - PubMed
Devoy 1995
-
- Devoy MAB, Fuller RW, Palmer JBD. Asthma mortality and beta agonists. Chest 1995;108:1768. - PubMed
Devoy Fuller 1995
-
- Devoy MAB, Fuller RW, Palmer JBD. Beta agonists in the treatment of asthma?. Chest 1995;107:1116‐24. - PubMed
Faurschou 1994
-
- Faurschou P, Engel AM, Haanaes OC. [Salmeterol in two different doses in the treatment of nocturnal bronchial asthma poorly controlled by other therapies]. Allergy 1994;10:827‐32. - PubMed
French 1994
-
- French DJ, Christie MJ, Sowden AJ. The reproducibility of the childhood asthma Questionnaires: measures of quality of life for children with asthma aged 4‐16 yrs. Quality of Life Research 1994;3:215‐24. - PubMed
Fuller 1995
-
- Fuller R. Safety of salmeterol in the treatment of asthma. European Respiratory Review 1995;5:133‐7.
Giannini 2000
-
- Giannini D, Franco A, Bacci E, Dente FL, Taccola M, Vagaggini B, et al. The protective effect of salbutamol inhaled using different devices on methacholine bronchoconstriction. Chest 2000;117(5):1319‐23. - PubMed
GINA 1995
-
- National heart Lung and Blood Institute/WHO. Global strategy for asthma management and prevention. Bethesda: National Institutes of Health, 1995. National heart Lung and Blood Institute/WHO Report. 1995.
Hasselblad 1995
-
- Hasselblad V, Mosteller F, Littenberg B, Chalmers TC, Hunink MG, Turner JA, et al. A survey of current problems in meta‐analysis. Discussion from the Agency for Health Care Policy and Research inter‐PORT Work Group on Literature Review/Meta‐Analysis. Med Care 1995;33(2):202‐20. - PubMed
Howarth 2000
-
- Howarth PH, Beckett P, Dahl R. The effect of long‐acting beta2‐agonists on airway inflammation in asthmatic patients. Respiratory Medicine 2000;94 Supp (F):22‐25. - PubMed
Hyland 1991
-
- Hyland M. The living with asthma questionnaire. Respir Med 1991;85 Suppl:13‐6. - PubMed
Jadad 1996
-
- Jadad A, Moore R, Carroll D, et al. [Assessing the quality of reports of randomised control trials: is blinding really neccesary?]. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Janson 2001
-
- Janson C, Anto J, Burney P, et al. The European Community Respiratory Health Survey: what are the main results so far? European Community Respiratory Health Survey II. European Respiratory Journal 2001;18:598‐611. - PubMed
Juniper 1992
Juniper 1994
-
- Juniper EF, Guyatt GH, Willan A, Griffith L. [Determining a minimal important change in a disease‐specific quality of life questionnaire]. Journal of Clinical Epidemiology 1994;47:81‐7. - PubMed
Kips 2001
-
- Kips J, Pauwels R. Long‐acting inhaled best2agonist therapy in asthma. American Journal of Respiratory & Critical Care Medicine 2001;164:923‐32. - PubMed
Korosec 1999
-
- Korosec M, Novak D, Myers E, Skrowonski M, McFadden EJ. [Salmeterol does not compromise the bronchodilator response to albuterol during acute episodes of asthma]. American Journal of Medicine 1999;107:209‐13. - PubMed
Lai 1989
-
- Lai CKW, Twentyman OP, Holgate ST. The effect of an increase in inhaled allergen dose after rimiterol hydrobromide on the occurrence and magnitude of the late asthmatic response and the associated change in nonspecific bronchial hyperresponsiveness. American Review of Respiratory Disease 1989;140:917‐23. - PubMed
Lipworth 1999
-
- Lipworth B, Hall I, Tan S, Aziz I, Coutie W. Effects of genetic polymorphism on ex vivo and in vivo function of beta2‐adrenoceptors in asthmatic patients. Chest 1999;115:324‐8. - PubMed
Lipworth Demp 2000
-
- Lipworth BJ, Dempsey OJ, Aziz I. Functional antagonism with formoterol and salmeterol in asthmatic patients expressing the homozygous Glycine‐16 beta2 adrenoceptor polmorphism. Chest 2000;118(2):321‐8. - PubMed
Lipworth Hall 1999
-
- Lipworth BJ, Hall IP, Aziz I, Tan KS, Wheatley A. Beta2‐adrenoceptor polymorphism and bronchoprotective sensitivity with regular short and long‐acting beta2‐agonist therapy. Clin Sci (Colch) 1999;96:253‐9. - PubMed
Moore 1996
-
- Moore RH, Khan A, Dickey B. long acting inhaled beta 2 agonists in asthma therapy. Chest 1996;113:1095‐108. - PubMed
NAC 1998
-
- National Asthma Campaign. Asthma Management Handbook. 1998.
Page 1993
-
- Page CP. An explanation of the asthma paradox. American Review of Respiratory Disease 1993;147 Suppl:29‐33. - PubMed
Palmquist 1997
-
- Palmquist M, Persson G, Lazer L, Rosenborg J, Larsson P, Lotvall J. Inhaled dry‐powder formoterol and slameterol in asthmatic patients: onset of action, duration of effect and potency. European Respiratory Journal 1997;10:2484‐9. - PubMed
Partridge 2000
-
- Partridge M, Fabbri L, Chung K. Delivering effective asthma care‐ how do we implement asthma guidelines?. European Respiratory Journal 2000;15:235‐7. - PubMed
Pearce 2000
-
- Pearce N, Sunyer J, Cheng S, et al. Comparison of asthma prevalence in the ISAAC and the ECRHS. ISAAC Steering Committee and the European Community Respiratory Health Survey. International Study of Asthma and Allergies in Childhood.. European Respiratory Journal 2000;16(3):420‐6. - PubMed
Sears 1998
-
- Sears MR. Asthma treatment: inhaled beta‐agonists [Review]. Canadian Respiratory Journal 1998;5:54‐9A. - PubMed
Sears Rea 1986
Selroos 1998
-
- Selroos O. The pharmacologic and clinical properties of Oxis (formoterol) Turbuhaler. Allergy 1998;53(42 Suppl):14‐9. - PubMed
Shale 1994
-
- Shale DJ. Respiratory arrests in young asthmatics on salmeterol. Respiratory Medicine 1994;88:75‐6. - PubMed
Spitzer 1992
-
- Spitzer WO, Suissa S, Ernst P, Horwitz RI, Habbick B, et al. The use of beta agonists and the risk of death and near death from asthma. New England Journal of Medicine 1992;326:501‐6. - PubMed
Sullivan 1996
-
- Sullivan S, Elixhauser A, Buist A, Luce B, Eisenberg J, Weiss K. National Asthma Education and Prevention Program working group report on the cost effectiveness of asthma care Program working group report on the cost effectiveness of asthma care. American Journal of Respiratory & Critical Care Medicine 1996;154 Suppl:85‐95. - PubMed
Tan Hall 1997
-
- Tan KS, Hall IP, Dewar JC, Dow E, Lipworth BJ. Association between beta2‐adrenoceptor polymorphism and susceptibility to bronchodilator desensitisation in moderately severe stable asthmatics. Lancet 1997;350:995‐9. - PubMed
Tattersfield 1993
-
- Tattersfield AE. Clinical pharmacology of long‐acting beta‐receptor agonists. [review]. Life Sciences 1993;52(26):2151‐9. - PubMed
Taylor 1997
-
- Taylor D, Jensen M, Aikman S, Harris J, Barnes PJ, O'Connor B. Comparison of salmeterol and albuterol induced bronchoprotection against AMP and histamine in mild asthma. American Journal of Respiratory & Critical Care Medicine 1997;156:1731‐7. - PubMed
Taylor 2000
TSANZ 1989
-
- Thoracic Society Australia & NZ. Asthma management plan. Medical Journal of Australia 1989;15:650‐3. - PubMed
Van der Molen 1997
-
- Molen T, Postma DS, Schreurs AJM, Bosveld HEP, Sears MR, Meyboom de Jong B. Discriminative aspects of two generic and two asthma‐specific instruments: Relation with symptoms, bronchodilator use and lung function in patients with mild asthma. Quality of Life Research 1997;6(4):353‐61. - PubMed
van Noord 1996
-
- Noord J, Smeets J, Raaijmakers J, Bommer a, Maesen F. Salmeterol versus formoterol in patients with moderately severe asthma: onset and duration of action. European Respiratory Journal 1996;9:1684‐8. - PubMed
Van Schayck 2000
-
- Schayck C. Do long‐acting beta2‐adrenergic agonists deserve a different place in guidelines for the treatment of asthma and COPD?. European Respiratory Journal 2000;15(4):631‐4. - PubMed
Verberne 1995
-
- Verberne AAPH, McCormack EM, Fuller R, et al. An overview of nine clinical trials of salmeterol in an asthmatic population. European Respiratory Journal 1995;8 Suppl (19)(suppl 19):156. - PubMed
Vervloet 1998
-
- Vervloet D, Ekstrom T, Pela R, Duce Gracia F, Kopp C, Silvert BD, et al. A 6‐month comparison between formoterol and salmeterol in patients with reversible obstructive airways disease. Respiratory Medicine 1998;92(6):836‐42. - PubMed
Walters 1999
-
- Walters EH, Walters J. Inhaled short acting beta‐2 agonist use in asthma: Regular versus as‐needed treatment. The Cochrane Library 1999, Issue 1. - PubMed
Weinberger 1995
-
- Weinberger M. Salmeterol for the treatment of asthma. Annals of Allergy, Asthma, & Immunology 1995;75:209‐11. - PubMed
Woods 2001
-
- Woods R, Walters E, Wharton C, Watson N, M Abramson. The rising prevalence of asthma in young Melbourne adults is associated with improvement in treatment. Annals of Allergy, Asthma, & Immunology 2001;87:117‐23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

