Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay
- PMID: 12522048
- PMCID: PMC145272
- DOI: 10.1128/cdli.10.1.108-115.2003
Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay
Abstract
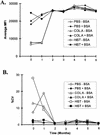
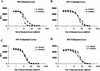
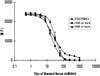
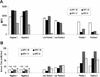
Several different methods have been developed to quantitate neutralizing antibody responses to human papillomaviruses (HPVs), including in vivo neutralization assays, in vitro pseudoneutralization assays, competitive radioimmunoassays (cRIAs), and enzyme-linked immunosorbent assays. However, each of these techniques possesses one or more limitations that preclude testing large numbers of patient sera for use in natural history studies and large vaccine clinical trials. We describe here a new multiplexed assay, by using the Luminex Laboratory MultiAnalyte Profiling (LabMAP3) assay system, that can simultaneously quantitate neutralizing antibodies to human papillomavirus types 6, 11, 16, and 18 in 50 micro l of serum. The HPV-Luminex competitive immunoassay measures titers of polyclonal antibodies in serum capable of displacing phycoerythrin-labeled detection monoclonal antibodies binding to conformationally sensitive, neutralizing epitopes on the respective virus-like particles. This competitive Luminex immunoassay was found to be as sensitive, accurate, and precise as the currently used cRIAs. An effective HPV vaccine will most likely require several distinct genotypes to protect against multiple cancer causing papillomaviruses. The HPV-Luminex immunoassay should prove to be a useful tool in simultaneously quantitating antibody immune responses to multiple HPV genotypes for natural history infection studies and for monitoring the efficacy of prospective vaccines.
Figures


Similar articles
-
Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18.Clin Diagn Lab Immunol. 2005 Aug;12(8):959-69. doi: 10.1128/CDLI.12.8.959-969.2005. Clin Diagn Lab Immunol. 2005. PMID: 16085914 Free PMC article.
-
Development of a human papillomavirus competitive luminex immunoassay for 9 HPV types.Hum Vaccin Immunother. 2014;10(8):2168-74. doi: 10.4161/hv.29205. Hum Vaccin Immunother. 2014. PMID: 25424920 Free PMC article. Review.
-
Evolution of type-specific immunoassays to evaluate the functional immune response to Gardasil: a vaccine for human papillomavirus types 16, 18, 6 and 11.Hum Vaccin. 2008 Mar-Apr;4(2):134-42. doi: 10.4161/hv.4.2.5261. Epub 2007 Nov 4. Hum Vaccin. 2008. PMID: 18388490
-
The humoral response to Gardasil over four years as defined by total IgG and competitive Luminex immunoassay.Hum Vaccin. 2011 Feb;7(2):230-8. doi: 10.4161/hv.7.2.13948. Epub 2011 Feb 1. Hum Vaccin. 2011. PMID: 21307649
-
Papillomavirus-like particles for serology and vaccine development.Intervirology. 1996;39(1-2):54-61. doi: 10.1159/000150475. Intervirology. 1996. PMID: 8957670 Review.
Cited by
-
Comparison of different assays to assess human papillomavirus (HPV) type 16- and 18-specific antibodies after HPV infection and vaccination.Clin Vaccine Immunol. 2013 Aug;20(8):1329-32. doi: 10.1128/CVI.00153-13. Epub 2013 Jun 5. Clin Vaccine Immunol. 2013. PMID: 23740920 Free PMC article.
-
Immunogenicity and safety of the human papillomavirus 6, 11, 16, 18 vaccine in HIV-infected young women.Clin Infect Dis. 2013 Sep;57(5):735-44. doi: 10.1093/cid/cit319. Epub 2013 May 10. Clin Infect Dis. 2013. PMID: 23667266 Free PMC article. Clinical Trial.
-
Inflammatory protein profiling of pancreatic cyst fluid using EUS-FNA in tandem with cytokine microarray differentiates between branch duct IPMN and inflammatory cysts.J Immunol Methods. 2012 Aug 31;382(1-2):142-149. doi: 10.1016/j.jim.2012.05.018. Epub 2012 Jun 7. J Immunol Methods. 2012. PMID: 22683544 Free PMC article.
-
Immunogenicity of a Quadrivalent Human Papillomavirus Vaccine When Co-Administered with Tetanus-Reduced Diphtheria-Acellular Pertussis and Quadrivalent Meningococcal Conjugate Vaccines in Healthy Adolescents: Results from a Randomized, Observer-Blind, Controlled Trial.Infect Dis Ther. 2019 Sep;8(3):335-341. doi: 10.1007/s40121-019-00258-5. Epub 2019 Aug 3. Infect Dis Ther. 2019. PMID: 31377946 Free PMC article.
-
Multiplexed VeraCode bead-based serological immunoassay for colorectal cancer.J Immunol Methods. 2013 Dec 31;400-401:58-69. doi: 10.1016/j.jim.2013.09.013. Epub 2013 Oct 24. J Immunol Methods. 2013. PMID: 24161315 Free PMC article.
References
-
- Bellisario, R., R. J. Colinas, and K. A. Pass. 2001. Simultaneous measurement of antibodies to three HIV-1 antigens in newborn dried blood-spot specimens using a multiplexed microsphere-based immunoassay. Early Hum. Dev. 64:21-25. - PubMed
-
- Bosch, F. X., M. M. Manos, N. Munoz, M. Sherman, A. M. Jansen, J. Peto, M. H. Schiffman, V. Moreno, R. Kurman, and K. V. Shah. 1995. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J. Natl. Cancer Inst. 87:796-802. - PubMed
-
- Breitburd, F., and P. Coursaget. 1999. Human papillomavirus vaccines. Semin. Cancer Biol. 9:431-444. - PubMed
-
- Breitburd, F., R. Kirnbauer, N. L. Hubbert, B. Nonnenmacher, C. Trin-Dinh-Desmarquet, G. Orth, J. T. Schiller, and D. R. Lowy. 1995. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 69:3959-3963. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

