Identification of B- and T-cell epitopes of BB, a carrier protein derived from the G protein of Streptococcus strain G148
- PMID: 12522050
- PMCID: PMC145282
- DOI: 10.1128/cdli.10.1.125-132.2003
Identification of B- and T-cell epitopes of BB, a carrier protein derived from the G protein of Streptococcus strain G148
Abstract
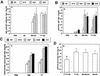
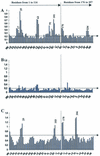
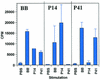
Most conventional vaccines consist of killed organisms or purified antigenic proteins. Such molecules are generally poorly immunogenic and need to be coupled to carrier proteins. We have identified a new carrier molecule, BB, derived from the G protein of Streptococcus strain G148. We show that BB is able to induce strong antibody responses when conjugated to peptides or polysaccharides. In order to localize T and B cell epitopes in BB and match them with the albumin-binding region of the molecule, we immunized mice with BB, performed B and T pepscan analyses, and compared the results with pepscan done with sera and cells from humans. Our results indicate that BB has two distinct T helper epitopes, seven linear B-cell epitopes, and one conformational B-cell epitope in BALB/c mice. Four linear B-cell epitopes were identified from human sera, three of which overlapped mouse B-cell epitopes. Finally, three human T-cell epitopes were detected on the BB protein. One of these T-cell epitopes is common to BALB/c mice and humans and was localized in the region that contains the albumin-binding site. These data are of interest for the optimization of new carrier molecules derived from BB.
Figures



Similar articles
-
Identification of B- and T-cell epitopes within the fibronectin-binding domain of the SfbI protein of Streptococcus pyogenes.Infect Immun. 2003 Dec;71(12):7197-201. doi: 10.1128/IAI.71.12.7197-7201.2003. Infect Immun. 2003. PMID: 14638816 Free PMC article.
-
Identifying B and T cell epitopes and studying humoral, mucosal and cellular immune responses of peptides derived from V antigen of Yersinia pestis.Vaccine. 2008 Jan 17;26(3):316-32. doi: 10.1016/j.vaccine.2007.11.028. Epub 2007 Dec 3. Vaccine. 2008. PMID: 18096277
-
A 10-amino-acid linear sequence of VP1 of foot and mouth disease virus containing B- and T-cell epitopes induces protection in mice.Virology. 1995 Oct 1;212(2):614-21. doi: 10.1006/viro.1995.1519. Virology. 1995. PMID: 7571431
-
Identification of murine B-cell and T-cell epitopes of Escherichia coli outer membrane protein F with synthetic polypeptides.Infect Immun. 2000 May;68(5):2535-45. doi: 10.1128/IAI.68.5.2535-2545.2000. Infect Immun. 2000. PMID: 10768941 Free PMC article.
-
Molecular analysis of human cardiac myosin-cross-reactive B- and T-cell epitopes of the group A streptococcal M5 protein.Infect Immun. 1997 Sep;65(9):3913-23. doi: 10.1128/iai.65.9.3913-3923.1997. Infect Immun. 1997. PMID: 9284171 Free PMC article.
Cited by
-
Early implementation of QbD in biopharmaceutical development: a practical example.Biomed Res Int. 2015;2015:605427. doi: 10.1155/2015/605427. Epub 2015 May 17. Biomed Res Int. 2015. PMID: 26075248 Free PMC article. Review.
-
ABD-Derived Protein Blockers of Human IL-17 Receptor A as Non-IgG Alternatives for Modulation of IL-17-Dependent Pro-Inflammatory Axis.Int J Mol Sci. 2018 Oct 9;19(10):3089. doi: 10.3390/ijms19103089. Int J Mol Sci. 2018. PMID: 30304852 Free PMC article.
-
Conjugate of Doxorubicin to Albumin-Binding Peptide Outperforms Aldoxorubicin.Small. 2019 Mar;15(12):e1804452. doi: 10.1002/smll.201804452. Epub 2019 Feb 13. Small. 2019. PMID: 30756483 Free PMC article.
References
-
- Alexander, J., M. F. del Guercio, A. Maewal, L. Qiao, J. Fikes, R. W. Chesnut, J. Paulson, D. R. Bundle, S. DeFrees, and A. Sette. 2000. Linear PADRE T helper epitope and carbohydrate B-cell epitope conjugates induce specific high titer IgG antibody responses. J. Immunol. 164:1625-1633. - PubMed
-
- Bjorck, L., W. Kastern, G. Lindahl, and K. Wideback. 1987. Streptococcal protein G, expressed by streptococci or by Escherichia coli, has separate binding sites for human albumin and IgG. Mol. Immunol. 24:1113-1122. - PubMed
-
- Bockenstedt, L. K., E. Fikrig, S. W. Barthold, R. A. Flavell, and F. S. Kantor. 1996. Identification of a Borrelia burgdorferi OspA T-cell epitope that promotes anti-OspA IgG in mice. J. Immunol. 157:5496-5502. - PubMed
-
- Dalum, I., M. R. Jensen, P. Hindersson, H. I. Elsner, and S. Mouritsen. 1996. Breaking of B cell tolerance toward a highly conserved self protein. J. Immunol. 157:4796-4804. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

