Regulation of muscarinic receptor function in developing oligodendrocytes by agonist exposure
- PMID: 12522072
- PMCID: PMC1573629
- DOI: 10.1038/sj.bjp.0705002
Regulation of muscarinic receptor function in developing oligodendrocytes by agonist exposure
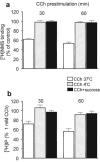
Abstract
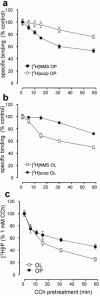
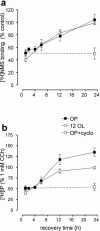
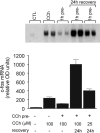
1 Oligodendrocytes, the myelin forming cells in the CNS, express muscarinic acetylcholine receptors (mAChR), primarily M3, coupled to various signal transduction pathways. 2 In the present study we have investigated whether mAChR undergo functional agonist-induced regulation in cultured oligodendrocyte progenitors and differentiated oligodendrocytes. 3 The muscarinic agonist, carbachol (CCh) caused a time-dependent desensitization of phosphoinositide (PI) hydrolysis, and the internalization and down-regulation of receptors. Short-time desensitization (5 min) of PI hydrolysis occurred without receptor internalization and reached 54% by 1 h. The same treatment decreased cell surface receptors labelled with the non-permeable ligand [(3)H]-NMS by 47%, while total receptor density ([(3)H]-scopolamine binding) decreased by 30%. Longer CCh treatment down-regulated receptors by 70% and desensitized the PI response by 80%. 4 Although protein kinase C (PKC) activation desensitized mAChR, CCh-mediated desensitization was independent of PKC. 5 Inhibition of receptor endocytosis by low temperature during the pre-stimulation period or in the presence of hyperosmotic sucrose (0.5 M) blocked desensitization, receptor internalization and down-regulation. 6 Recovery of surface mAChR and their functional activity following down-regulation was slow, returning to control levels by 24 h after agonist removal. In progenitor cells, dose-response curves for CCh-mediated PI hydrolysis and c-fos mRNA expression showed that newly synthesized mAChR were supersensitive after recovery. 7 Overall, the present results provide evidence of functional agonist-mediated mAChR regulation in brain oligodendroglial cells.
Figures



Similar articles
-
Mutation of carboxyl-terminal threonine residues in human m3 muscarinic acetylcholine receptor modulates the extent of sequestration and desensitization.Mol Pharmacol. 1995 Sep;48(3):477-85. Mol Pharmacol. 1995. PMID: 7565628
-
Effects of agonist efficacy on desensitization of phosphoinositide hydrolysis mediated by m1 and m3 muscarinic receptors expressed in Chinese hamster ovary cells.J Pharmacol Exp Ther. 1991 Jun;257(3):938-45. J Pharmacol Exp Ther. 1991. PMID: 1710663
-
Pharmacological and functional characterization of muscarinic receptor subtypes in developing oligodendrocytes.J Neurochem. 2001 Jun;77(5):1396-406. doi: 10.1046/j.1471-4159.2001.00356.x. J Neurochem. 2001. PMID: 11389190
-
Multiple mechanisms involving protein phosphorylation are linked to desensitization of muscarinic receptors.Life Sci. 1995;56(11-12):951-5. doi: 10.1016/0024-3205(95)00033-3. Life Sci. 1995. PMID: 10188798 Review.
-
Regulation of muscarinic acetylcholine receptor signaling.Pharmacol Ther. 2003 May;98(2):197-220. doi: 10.1016/s0163-7258(03)00032-9. Pharmacol Ther. 2003. PMID: 12725869 Review.
Cited by
-
Emerging Roles of Cholinergic Receptors in Schwann Cell Development and Plasticity.Biomedicines. 2022 Dec 24;11(1):41. doi: 10.3390/biomedicines11010041. Biomedicines. 2022. PMID: 36672549 Free PMC article. Review.
-
Systematic Review of Pharmacological Properties of the Oligodendrocyte Lineage.Front Cell Neurosci. 2016 Feb 12;10:27. doi: 10.3389/fncel.2016.00027. eCollection 2016. Front Cell Neurosci. 2016. PMID: 26903812 Free PMC article. Review.
-
Protein kinase C regulates the expression of M1 receptors and BDNF in rat retinal cells.Neurochem Res. 2009 May;34(5):884-90. doi: 10.1007/s11064-008-9847-x. Epub 2008 Sep 19. Neurochem Res. 2009. PMID: 18802750
-
Possible Correlation between Cholinergic System Alterations and Neuro/Inflammation in Multiple Sclerosis.Biomedicines. 2020 Jun 8;8(6):153. doi: 10.3390/biomedicines8060153. Biomedicines. 2020. PMID: 32521719 Free PMC article. Review.
-
Evaluation of in vitro neuronal platforms as surrogates for in vivo whole brain systems.Sci Rep. 2018 Jul 17;8(1):10820. doi: 10.1038/s41598-018-28950-5. Sci Rep. 2018. PMID: 30018409 Free PMC article.
References
-
- ALMAZAN G., AFAR D.E.H., BELL J.C. Phosphorylation and disruption of intermediate filament proteins in oligodendrocyte precursor cultures treated with calyculin A. J. Neurosci. Res. 1993;36:163–172. - PubMed
-
- BELACHEW S., ROGISTER B., RIGO J.M., MALGRANGE B., MOONEN G. Neurotransmitter mediated regulation of CNS myelination: a review. Acta Neurologica Bélgica. 1999;1:21–31. - PubMed
-
- BERGLES D.E., ROBERTS J.D.B., SOMOGYI P., JAHR C.E. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. - PubMed
-
- CHAVKIN C., MCLAUGHLIN J.P., CELVER J.P. Regulation of opioid receptor function by chronic agonist exposure: constitutive activity and desensitization. Mol. Pharmacol. 2001;60:20–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

