Unproductive cleavage and the inactivation of protease-activated receptor-1 by trypsin in vascular endothelial cells
- PMID: 12522081
- PMCID: PMC1573634
- DOI: 10.1038/sj.bjp.0705008
Unproductive cleavage and the inactivation of protease-activated receptor-1 by trypsin in vascular endothelial cells
Abstract
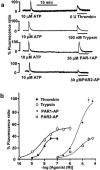
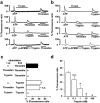
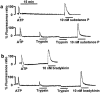
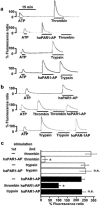
1 Using fura-2 fluorometry of [Ca(2+)](i) in response to thrombin, trypsin and protease-activated receptor activating peptides (PAR-APs), we determined whether trypsin cleaves protease-activated receptor 1 (PAR1) and activates it in the endothelial cells of the porcine aortic valves and human umbilical vein. 2 Once stimulated with thrombin, the subsequent application of trypsin induced a [Ca(2+)](i) elevation similar to that obtained without the preceding stimulation with thrombin in the valvular endothelial cells. However, the preceding stimulation with trypsin abolished the subsequent response to thrombin, but not to bradykinin or substance P. 3 The response to PAR1-AP (SFLLRNP) was significantly (P<0.05) reduced by the preceding stimulation with thrombin and PAR1-AP in the valvular endothelial cells, while, importantly, it remained unaffected by the preceding stimulation with either trypsin or PAR2-AP (SLIGRL). The response to PAR2-AP was reduced by the preceding stimulation with trypsin and PAP2-AP. PAR1-AP attenuated the subsequent responses not only to thrombin and PAR1-AP but also to trypsin and PAR2-AP, while PAR2-AP specifically attenuated the subsequent responses to trypsin and PAR2-AP. 4 In human umbilical vein endothelial cells, a higher affinity PAR1-AP (haPAR1-AP) (Ala-pF-Arg-Cha-HArg-Tyr-NH(2)) specifically attenuated the responses to thrombin but not trypsin. On the other hand, the response to haPAR1-AP was significantly (P<0.05) attenuated by the preceding stimulation with thrombin but not trypsin. 5 In conclusion, trypsin cleaved PAR1 but did not activate it in the endothelial cells. Moreover, the trypsin-cleaved PAR1 was no longer responsive to thrombin.
Figures



Similar articles
-
Inactivation of protease-activated receptor-1 by proteolytic removal of the ligand region in vascular endothelial cells.Biochem Pharmacol. 2004 Jul 1;68(1):23-32. doi: 10.1016/j.bcp.2004.03.005. Biochem Pharmacol. 2004. PMID: 15183114
-
Mechanism of trypsin-induced contraction in the rat myometrium: the possible involvement of a novel member of protease-activated receptor.Br J Pharmacol. 2001 Aug;133(8):1276-85. doi: 10.1038/sj.bjp.0704206. Br J Pharmacol. 2001. PMID: 11498513 Free PMC article.
-
Evaluation of proteinase-activated receptor-1 (PAR1) agonists and antagonists using a cultured cell receptor desensitization assay: activation of PAR2 by PAR1-targeted ligands.J Pharmacol Exp Ther. 1999 Jan;288(1):358-70. J Pharmacol Exp Ther. 1999. PMID: 9862790
-
Ion transport induced by proteinase-activated receptors (PAR2) in colon and airways.Cell Biochem Biophys. 2002;36(2-3):209-14. doi: 10.1385/CBB:36:2-3:209. Cell Biochem Biophys. 2002. PMID: 12139406 Review.
-
How the protease thrombin talks to cells.Proc Natl Acad Sci U S A. 1999 Sep 28;96(20):11023-7. doi: 10.1073/pnas.96.20.11023. Proc Natl Acad Sci U S A. 1999. PMID: 10500117 Free PMC article. Review.
Cited by
-
Optical biosensor differentiates signaling of endogenous PAR1 and PAR2 in A431 cells.BMC Cell Biol. 2007 Jun 22;8:24. doi: 10.1186/1471-2121-8-24. BMC Cell Biol. 2007. PMID: 17587449 Free PMC article.
-
Protease-activated receptors (PARs): mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases.Thromb J. 2019 Mar 29;17:4. doi: 10.1186/s12959-019-0194-8. eCollection 2019. Thromb J. 2019. PMID: 30976204 Free PMC article. Review.
-
Thrombin receptors and protease-activated receptor-2 in human placentation: receptor activation mediates extravillous trophoblast invasion in vitro.Am J Pathol. 2003 Oct;163(4):1245-54. doi: 10.1016/S0002-9440(10)63484-0. Am J Pathol. 2003. PMID: 14507634 Free PMC article.
-
Protease Activated Receptor 1 and Its Ligands as Main Regulators of the Regeneration of Peripheral Nerves.Biomolecules. 2021 Nov 10;11(11):1668. doi: 10.3390/biom11111668. Biomolecules. 2021. PMID: 34827666 Free PMC article. Review.
-
Impaired feedback regulation of the receptor activity and the myofilament Ca2+ sensitivity contributes to increased vascular reactiveness after subarachnoid hemorrhage.J Cereb Blood Flow Metab. 2010 Sep;30(9):1637-50. doi: 10.1038/jcbfm.2010.35. Epub 2010 Mar 17. J Cereb Blood Flow Metab. 2010. PMID: 20234381 Free PMC article.
References
-
- AHN H.S., FOSTER C., BOYKOW G., ARIK L., SMITH-TORHAN A., HESK D., CHATTERJEE M. Binding of a thrombin receptor tethered ligand analogue to human platelet thrombin receptor. Mol. Pharmacol. 1997;51:350–356. - PubMed
-
- BLACKHART B.D., EMILSSON K., NGUYEN D., TENG W., MARTELLI A.J., NYSTEDT S., SUNDELIN J., SCARBOROUGH R.M. Ligand cross-reactivity within the protease-activated receptor family. J. Biol. Chem. 1996;271:16466–16471. - PubMed
-
- BOHM S.K., KHITIN L.M., GRADY E.F., APONTE G., PAYAN D.G., BUNNETT N.W. Mechanisms of desensitization and resensitization of proteinase-activated receptor-2. J. Biol. Chem. 1996a;271:22003–22016. - PubMed
-
- COCKS T.M., MOFFATT J.D. Protease-activated receptors: sentries for inflammation. Trends. Pharmacol. Sci. 2000;21:103–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

