Butyrylcholinesterase and acetylcholinesterase activity and quantal transmitter release at normal and acetylcholinesterase knockout mouse neuromuscular junctions
- PMID: 12522088
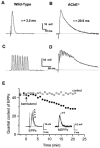
- PMCID: PMC1573636
- DOI: 10.1038/sj.bjp.0705010
Butyrylcholinesterase and acetylcholinesterase activity and quantal transmitter release at normal and acetylcholinesterase knockout mouse neuromuscular junctions
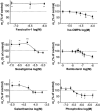
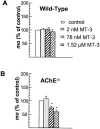
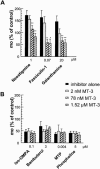
Abstract
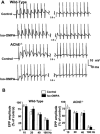
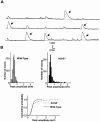
1 The present study was performed to evaluate the presence and the physiological consequences of butyrylcholinesterase (BChE) inhibition on isolated phrenic-hemidiaphragm preparations from normal mice expressing acetylcholinesterase (AChE) and BChE, and from AChE-knockout mice (AChE(-/-)) expressing only BChE. 2 Histochemical and enzymatic assays revealed abundance of AChE and BChE in normal mature neuromuscular junctions (NMJs). 3 In normal NMJs, in which release was reduced by low Ca(2+)/high Mg(2+) medium BChE inhibition with tetraisopropylpyrophosphoramide (iso-OMPA) or bambuterol decreased ( approximately 50%) evoked quantal release, while inhibition of AChE with fasciculin-1, galanthamine (10, 20 micro M) or neostigmine (0.1-1 micro M) increased (50-80%) evoked quantal release. Inhibition of both AChE and BChE with galanthamine (80 micro M), neostigmine (3-10 micro M), O-ethylS-2-(diisopropylamino)ethyl-methylphosphono-thioate (MTP) or phospholine decreased evoked transmitter release (20-50%). 4 In AChE(-/-) NMJs, iso-OMPA pre-treatment decreased evoked release. 5 Muscarinic toxin-3 decreased evoked release in both AChE(-/-) and normal NMJs treated with low concentrations of neostigmine, galanthamine or fasciculin-1, but had no effect in normal NMJs pretreated with iso-OMPA, bambuterol, MTP and phospholine. 6 In normal and AChE(-/-) NMJs pretreatment with iso-OMPA failed to affect the time course of miniature endplate potentials and full-sized endplate potentials. 7 Overall, our results suggest that inhibition or absence of AChE increases evoked quantal release by involving muscarinic receptors (mAChRs), while BChE inhibition decreases release through direct or indirect mechanisms not involving mAChRs. BChE apparently is not implicated in limiting the duration of acetylcholine action on postsynaptic receptors, but is involved in a presynaptic modulatory step of the release process.
Figures

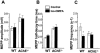
References
-
- ADLER M., FILBERT M.G. Role of butyrylcholinesterase in canine tracheal smooth muscle function. FEBS Lett. 1990;267:107–110. - PubMed
-
- ATACK J.R., YU Q.S., SONCRANT T.T., BROSSI A., RAPOPORT S.I. Comparative inhibitory effects of various physostigmine analogs against acetyl- and butyrylcholinesterases. J. Pharmacol. Exp. Ther. 1989;249:194–202. - PubMed
-
- BEHRA M., COUSIN X., BERTRAND C., VONESCH J.L., BIELLMANN D., CHATONNET A., STRÄHLE U. Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nat. Neurosci. 2002;5:111–118. - PubMed
-
- BOWMAN W.C., GIBB A.J., HARVEY A.L., MARSHALL I.G.Prejunctional actions of cholinoceptor agonist and antagonist and of anticholinesterase drugs Handbook of Experimental Pharmacology, Vol. 79, New neuromuscular Blocking Agents 1986Berlin: Springer-Verlag; 141–170.ed. Kharkevich, D.
-
- BOWMAN W.C., PRIOR C., MARSHALL I.G. Presynaptic receptors in the neuromuscular junction. Ann. N.Y. Acad. Sci. 1990;604:69–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

