Effector mechanism of magnolol-induced apoptosis in human lung squamous carcinoma CH27 cells
- PMID: 12522090
- PMCID: PMC1573650
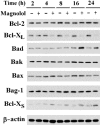
- DOI: 10.1038/sj.bjp.0705024
Effector mechanism of magnolol-induced apoptosis in human lung squamous carcinoma CH27 cells
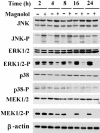
Abstract
1 Magnolol, an active component isolated from the root and stem bark of Magnolia officinalis, has been reported to exhibit antitumour effects, but little is known about its molecular mechanisms of action. 2 Magnolol inhibited proliferation of human lung squamous carcinoma CH27 cells at low concentrations (10-40 microM), and induced apoptosis at high concentrations (80-100 microM). 3 Treatment with 80 microM magnolol significantly increased the expression of Bad and Bcl-X(S) proteins, whereas it decreased the expression of Bcl-X(L). Overexpression of Bcl-2 protected CH27 cells against magnolol-triggered apoptosis. 4 Magnolol treatment resulted in accumulation of cytosolic cytochrome c and activation of caspase-9 and downstream caspases (caspase-3 and -6). Pretreatment with z-VAD-fmk markedly inhibited magnolol-induced cell death, but did not prevent cytosolic cytochrome c accumulation. 5 Magnolol induced a modest and persistent JNK activation and ERK inactivation in CH27 cells without evident changes in the protein levels. The responsiveness of JNK and ERK to magnolol suggests the involvement of these kinases in the initiation of the apoptosis process. 6 These results indicate that regulation of the Bcl-2 family, accumulation of cytosolic cytochrome c, and activation of caspase-9 and caspase-3 may be the effector mechanisms of magnolol-induced apoptosis.
Figures
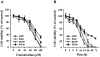




References
-
- ADAMS J.M., CORY S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. - PubMed
-
- BANG K.H., KIM Y.K., MIN B.S., NA M.K., RHEE Y.H., LEE J.P., BAE K.H. Antifungal activity of magnolol and honokiol. Arch. Pharm. Res. 2000;23:46–49. - PubMed
-
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. - PubMed
-
- CHANG W.S., CHANG Y.H., LU F.J., CHIANG H.C. Inhibitory effects of phenolics on xanthine oxidase. Anticancer Res. 1994;14:501–506. - PubMed
-
- CHEN C.J., YOU S.L., LIN L.H., HSU W.L., YANG Y.W. Cancer epidemiology and control in Taiwan: a brief review. Jpn J. Clin. Oncol. 2002;32:S66–S81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

