Comparison of signalling mechanisms involved in rat mesenteric microvessel contraction by noradrenaline and sphingosylphosphorylcholine
- PMID: 12522098
- PMCID: PMC1573654
- DOI: 10.1038/sj.bjp.0705028
Comparison of signalling mechanisms involved in rat mesenteric microvessel contraction by noradrenaline and sphingosylphosphorylcholine
Abstract
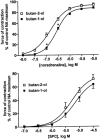
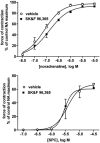
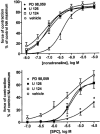
1 We have compared the signalling mechanisms involved in the pertussis toxin-sensitive and -insensitive contraction of rat isolated mesenteric microvessels elicited by sphingosylphosphorylcholine (SPC) and noradrenaline (NA), respectively. 2 The phospholipase D inhibitor butan-1-ol (0.3%), the store-operated Ca(2+) channel inhibitor SK>F 96,365 (10 microM), the tyrosine kinase inhibitor genistein (10 microM), and the src inhibitor PP2 (10 microM) as well as the negative controls (0.3% butan-2-ol and 10 microM diadzein and PP3) had only little effect against either agonist. 3 Inhibitors of phosphatidylinositol-3-kinase (wortmannin and LY 294,002, 10 microM each) or of mitogen-activated protein kinase kinase (PD 98,059 and U 126, 10 microM each) did not consistently attenuate NA- and SPC-induced contraction as compared to their vehicles or negative controls (LY 303,511 or U 124). 4 The phospholipase C inhibitor U 73,122 (10 microM) markedly inhibited the SPC- and NA-induced contraction (70% and 88% inhibition of the response to the highest NA and SPC concentration, respectively), whereas its negative control U 73,343 (10 microM) caused only less than 30% inhibition. 5 The rho-kinase inhibitors Y 27,632 (10 microM) and fasudil (30 microM) caused a rightward-shift of the NA concentration-response curve by 0.7-0.8 log units and reduced the response to 10 microM SPC by 88% and 83%, respectively. 6 These data suggest that SPC and NA, while acting on different receptors coupling to different G-protein classes, elicit contraction of rat mesenteric microvessels by similar signalling pathways including phospholipase C and rho-kinase.
Figures




Similar articles
-
Sphingosine-1-phosphate and sphingosylphosphorylcholine constrict renal and mesenteric microvessels in vitro.Br J Pharmacol. 2000 Aug;130(8):1871-7. doi: 10.1038/sj.bjp.0703515. Br J Pharmacol. 2000. PMID: 10952677 Free PMC article.
-
Comparison of contractile mechanisms of sphingosylphosphorylcholine and sphingosine-1-phosphate in rabbit coronary artery.Cardiovasc Res. 2009 May 1;82(2):324-32. doi: 10.1093/cvr/cvp054. Epub 2009 Feb 13. Cardiovasc Res. 2009. PMID: 19218288
-
Comparison of noradrenaline and lysosphingolipid-induced vasoconstriction in mouse and rat small mesenteric arteries.Auton Autacoid Pharmacol. 2004 Jul;24(3):77-85. doi: 10.1111/j.1474-8673.2004.00319.x. Auton Autacoid Pharmacol. 2004. PMID: 15541015
-
Pathophysiology of mesenteric ischemia.Surg Clin North Am. 1992 Feb;72(1):31-41. doi: 10.1016/s0039-6109(16)45626-4. Surg Clin North Am. 1992. PMID: 1731388 Review.
-
Noradrenaline transmission reducing drugs may protect against a broad range of diseases.Auton Autacoid Pharmacol. 2015 Apr;34(3-4):15-26. doi: 10.1111/aap.12019. Epub 2014 Oct 1. Auton Autacoid Pharmacol. 2015. PMID: 25271382 Review.
Cited by
-
Cardiovascular effects of sphingosine-1-phosphate and other sphingomyelin metabolites.Br J Pharmacol. 2004 Nov;143(6):666-84. doi: 10.1038/sj.bjp.0705934. Epub 2004 Oct 25. Br J Pharmacol. 2004. PMID: 15504747 Free PMC article. Review.
-
Sphingosylphosphorylcholine potentiates vasoreactivity and voltage-gated Ca2+ entry via NOX1 and reactive oxygen species.Cardiovasc Res. 2015 Apr 1;106(1):121-30. doi: 10.1093/cvr/cvv029. Epub 2015 Feb 6. Cardiovasc Res. 2015. PMID: 25661082 Free PMC article.
-
Augmented sphingosylphosphorylcholine-induced Ca2+-sensitization of mesenteric artery contraction in spontaneously hypertensive rat.Naunyn Schmiedebergs Arch Pharmacol. 2006 Apr;373(1):30-6. doi: 10.1007/s00210-006-0036-7. Epub 2006 Mar 7. Naunyn Schmiedebergs Arch Pharmacol. 2006. PMID: 16521007
-
Src family tyrosine kinases mediate contraction of rat isolated tail arteries in response to a hyposmotic stimulus.J Hypertens. 2007 Sep;25(9):1871-8. doi: 10.1097/HJH.0b013e328255e8f0. J Hypertens. 2007. PMID: 17762651 Free PMC article.
-
Phospholipase C-delta1 modulates sustained contraction of rat mesenteric small arteries in response to noradrenaline, but not endothelin-1.Am J Physiol Heart Circ Physiol. 2008 Aug;295(2):H826-34. doi: 10.1152/ajpheart.01396.2007. Epub 2008 Jun 20. Am J Physiol Heart Circ Physiol. 2008. PMID: 18567701 Free PMC article.
References
-
- ABURTO T., JINSI A., ZHU Q., DETH R.C. Involvement of protein kinase C activation in α2-adrenoceptor-mediated contractions of rabbit saphenous vein. Eur. J. Pharmacol. 1995;277:35–44. - PubMed
-
- ALLEN B.G., WALSH M.P. The biochemical basis of the regulation of smooth-muscle contraction. Trends Biochem. Sci. 1994;19:362–368. - PubMed
-
- ANDERSON R.A., BORONENKOV I.V., DOUGHMAN S.D., KUNZ J., LOIJENS J.C. Phosphatidylinositol phosphate kinases, a multifaceted family of signaling enzymes. J. Biol. Chem. 1999;274:9907–9910. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

