Intravascular adenoviral agents in cancer patients: lessons from clinical trials
- PMID: 12522437
- PMCID: PMC7091735
- DOI: 10.1038/sj.cgt.7700539
Intravascular adenoviral agents in cancer patients: lessons from clinical trials
Abstract
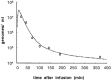
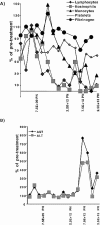
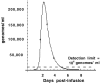
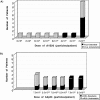
A large number of adenoviral agents are being developed for the treatment of cancer. However, the treatment-related death of a patient with ornithine transcarbamylase deficiency following adenovirus administration by hepatic artery has led to serious concerns regarding the safety of intravascular adenovirus. Both replication-incompetent (rAd.p53, e.g., SCH58500) and replication-selective (dl1520, aka Onyx-015; CG7870) oncolytic adenoviruses, by intravascular administration, are in clinical trials. We review Phases I and I/II results from these clinical trials. dl1520 and rAd.p53 were well-tolerated following hepatic artery infusion at doses of up to 2x10(12) and 2.5x10(13) particles, respectively. At a dose of 7.5x10(13) particles, rAd.p53 was associated with dose-limiting cardiac output suppression; dl1520 dose escalation did not proceed higher than 2x10(12). Intravenous (i.v.) infusions of dl1520 and CG7870 have been well tolerated by i.v. infusion at doses of 2x10(13) and 6x10(12), respectively, without identification of a maximally tolerated dose to date. Mild/moderate transaminitis was demonstrated in some patients on both the hepatic arterial and i.v. trials at doses >or=10(12) particles. Interleukin (IL)-6 and IL-10 were induced in a dose-dependent manner in most patients, but significant interpatient and intrapatient (on repeat doses) variabilities were demonstrated. Evidence of p53 gene expression (Ad.p53) or viral replication (dl1520) was demonstrated in the majority of patients receiving >or=10(12) particles. Over 100 cancer patients have been treated with intravascular adenovirus constructs to date with an acceptable toxicity profile; further clinical trial testing appears appropriate in cancer patients.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

