Cyclic AMP is sufficient for triggering the exocytic recruitment of aquaporin-2 in renal epithelial cells
- PMID: 12524527
- PMCID: PMC1315811
- DOI: 10.1038/sj.embor.embor711
Cyclic AMP is sufficient for triggering the exocytic recruitment of aquaporin-2 in renal epithelial cells
Abstract
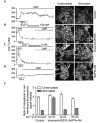
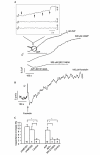
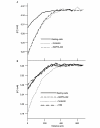
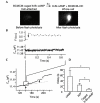
The initial response of renal epithelial cells to the antidiuretic hormone arginine vasopressin (AVP) is an increase in cyclic AMP. By applying immunofluorescence, cell membrane capacitance and transepithelial water flux measurements we show that cAMP alone is sufficient to elicit the antidiuretic cellular response in primary cultured epithelial cells from renal inner medulla, namely the transport of aquaporin-2 (AQP2)-bearing vesicles to, and their subsequent fusion with, the plasma membrane (AQP2 shuttle). The AQP2 shuttle is evoked neither by AVP-independent Ca(2+) increases nor by AVP-induced Ca(2+) increases. However, clamping cytosolic Ca(2+) concentrations below resting levels at 25 nM inhibited exocytosis. Exocytosis was confined to a slow monophasic response, and readily releasable vesicles were missing. Analysis of endocytic capacitance steps revealed that cAMP does not decelerate the retrieval of AQP2 from the plasma membrane. Our data suggest that cAMP initiates an early step, namely the transport of AQP2-bearing vesicles towards the plasma membrane, and do not support a regulatory function for Ca(2+) in the AQP2 shuttle.
Figures
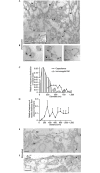
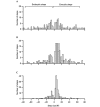
References
-
- Agre P., Nielsen S. & Knepper M.A. (2000) in The Kidney. Physiology and Pathophysiology (ed. Seldin, D.W. & Giebisch, G.) 363–377. Lippincott Williams & Wilkins, Philadelphia.
-
- Chou C.L. et al. . (2000) Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct—roles of ryanodinesensitive Ca2+ stores and calmodulin. J. Biol. Chem., 275, 36839–36846. - PubMed
-
- Deen P.M.T. et al. . (1994) Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science, 264, 92–95. - PubMed
-
- Hacker G.W. et al. . (1988) Silver acetate autometallography: an alternative enhancement technique for immunogoldsilver staining (IGSS) and silver amplification of gold, silver, mercury, and zinc in tissues. J. Histotechnol., 11, 213–221.
-
- Hagen V. et al. . (2001) Highly efficient and ultrafast phototriggers for cAMP and cGMP by using long-wavelength UV/VIS-activation. Angew. Chem. Int. Ed., 40, 1045–1048. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

