Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy
- PMID: 12525428
- PMCID: PMC145299
- DOI: 10.1128/CMR.16.1.114-128.2003
Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy
Abstract
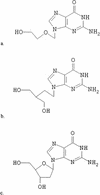
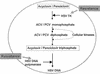
Acyclovir, penciclovir, and their prodrugs have been widely used during the past two decades for the treatment of herpesvirus infections. In spite of the distribution of over 2.3 x 10(6) kg of these nucleoside analogues, the prevalence of acyclovir resistance in herpes simplex virus isolates from immunocompetent hosts has remained stable at approximately 0.3%. In immuncompromised patients, in whom the risk for developing resistance is much greater, the prevalence of resistant virus has also remained stable but at a higher level, typically 4 to 7%. These observations are examined in the light of characteristics of the virus, the drugs, and host factors.
Figures

References
-
- Anderson, H., J. H. Scarffe, R. N. P. Sutton, E. Hickmott, D. Bridgen, and C. Burke. 1984. Oral acyclovir prophylaxis against herpes simplex virus in non-Hodgkin lymphoma and acute lymphoblastic leukaemia patients receiving remission induction chemotherapy. A randomised double blind, placebo controlled trial. Br. J. Cancer 50:45-49. - PMC - PubMed
-
- Bader C., C. S. Crumpacker, L. E. Schnipper, B. Ransil, J. E. Clark, K. Arndt, and I. M. Freedberg. 1978. The natural history of recurrent facial-oral infection with herpes simplex virus. J. Infect. Dis. 138:897-905. - PubMed
-
- Barry, D. W., S. Nusinoff-Lehrman, M. N. Ellis, K. K. Biron, and P. A. Furman. 1985. Viral resistance, clinical experience. Scand. J. Infect. Dis. 47(Suppl):155-164. - PubMed
-
- Beauchamp L. M., G. F. Orr, P. de Miranda, T. Burnette, and T. A. Krenitsky. 1992. Amino acid ester prodrugs of acyclovir. Antiviral Chem. Chemother. 3:157-164.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

