The natural host range shift and subsequent evolution of canine parvovirus resulted from virus-specific binding to the canine transferrin receptor
- PMID: 12525605
- PMCID: PMC140992
- DOI: 10.1128/jvi.77.3.1718-1726.2003
The natural host range shift and subsequent evolution of canine parvovirus resulted from virus-specific binding to the canine transferrin receptor
Abstract
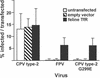
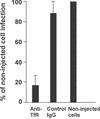
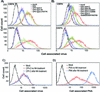
Canine parvovirus (CPV) is a host range variant of a feline virus that acquired the ability to infect dogs through changes in its capsid protein. Canine and feline viruses both use the feline transferrin receptor (TfR) to infect feline cells, and here we show that CPV infects canine cells through its ability to specifically bind the canine TfR. Receptor binding on host cells at 37 degrees C only partially correlated with the host ranges of the viruses, and an intermediate virus strain (CPV type 2) bound to higher levels on cells than did either the feline panleukopenia virus or a later strain of CPV. During the process of adaptation to dogs the later variant strain of CPV gained the ability to more efficiently use the canine TfR for infection and also showed reduced binding to feline and canine cells compared to CPV type 2. Differences on the top and the side of the threefold spike of the capsid surface controlled specific TfR binding and the efficiency of binding to feline and canine cells, and these differences also determined the cell infection properties of the viruses.
Figures




References
-
- Agbandje, M., R. McKenna, M. G. Rossmann, M. L. Strassheim, and C. R. Parrish. 1993. Structure determination of feline panleukopenia virus empty particles. Proteins 16:155-171. - PubMed
-
- Agbandje-McKenna, M., A. L. Llamas-Saiz, F. Wang, P. Tattersall, and M. G. Rossmann. 1998. Functional implications of the structure of the murine parvovirus, minute virus of mice. Structure 6:1369-1381. - PubMed
-
- Barbis, D. P., S.-F. Chang, and C. R. Parrish. 1992. Mutations adjacent to the dimple of canine parvovirus capsid structure affect sialic acid binding. Virology 191:301-308. - PubMed
-
- Bates, G. W., and M. R. Schlabach. 1973. The reaction of ferric salts with transferrin. J. Biol. Chem. 248:3228-3232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

