Human papillomavirus type 16 E2 protein has no effect on transcription from episomal viral DNA
- PMID: 12525636
- PMCID: PMC140940
- DOI: 10.1128/jvi.77.3.2021-2028.2003
Human papillomavirus type 16 E2 protein has no effect on transcription from episomal viral DNA
Abstract
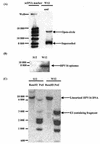
The human papillomavirus (HPV) E2 protein plays an important role in viral DNA replication. Many studies with high-risk HPVs have demonstrated that the E2 protein can also repress transcription of the E6 and E7 oncogenes. This conclusion, based on experiments carried out with cervical cancer cells bearing integrated HPV genomes, is currently assumed to be applicable to the normal HPV life cycle, in which the viral genomes are episomal. Here, we have tested experimentally whether this assumption is correct. We made use of a pair of isogenic cell lines, W12 and S12. W12 cells contain episomal HPV16 genomes, whereas S12 cells, which are derived from the W12 line, contain HPV DNA as integrated copies. When we expressed E2 in S12 cells, we observed strong repression of E6 and E7 transcription. In contrast, no effect of E2 on the transcription of these genes was detected in W12 cells. While integration of the viral genome into the host DNA contributes to the difference between W12 and S12 cells, integration by itself is not sufficient to explain this difference. Instead, the chromatin structure in the region of the E6 and E7 promoter (p97), which we show to be very different in these two cell lines, is likely to be the cause of the different responsiveness of p97 to the E2 protein. Experiments with the histone deacetylase inhibitor trichostatin A (TSA) indicated that the episomal HPV16 DNA is in a relatively inaccessible state prior to TSA treatment. Our results, together with those of others, suggest that any effect of the E2 protein on the expression of the E6 and E7 genes during the normal viral life cycle is of secondary importance compared to the function of E2 in replication.
Figures
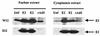


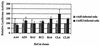

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

