Enhancement of antibodies to the human immunodeficiency virus type 1 envelope by using the molecular adjuvant C3d
- PMID: 12525639
- PMCID: PMC140896
- DOI: 10.1128/jvi.77.3.2046-2055.2003
Enhancement of antibodies to the human immunodeficiency virus type 1 envelope by using the molecular adjuvant C3d
Abstract
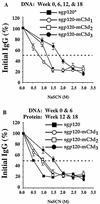
DNA vaccines expressing the envelope (Env) protein of the human immunodeficiency virus have been relatively ineffective at generating high-titer, long-lasting, neutralizing antibodies in a variety of animal models. In this study, the murine and human homologues of the complement component, C3d, were used in a DNA vaccine to enhance the titers of antibody to Env. Initially, plasmids expressing a secreted form of Env (sgp120) fused to one, two, or three copies of the murine homologue of C3d (mC3d) were constructed. Mice were inoculated with four vaccinations of DNA or two DNA vaccinations, followed by two boosts of affinity-purified gp120 protein. Analyses of titers demonstrated that multiple copies of mC3d coupled to sgp120 induced long-lasting, high-titer anti-Env antibody. Priming mice with sgp120-mC3d-DNA, followed by inoculation of purified gp120 protein, elicited the strongest antibody titers; however, the avidity maturation of the antibody was accelerated in the mice inoculated with sgp120-mC3d(3)-DNA. In addition, DNAs expressing sgp120 fused to three copies of the human homologue of C3d (hC3d(3)) efficiently enhanced the anti-Env antibody in rabbits. Lastly, antisera from both mice and rabbits vaccinated with DNA expressing sgp120-C3d(3) elicited higher titers of neutralizing antibody than did nonfused forms of Env. These results indicate that C3d, conjugated to sgp120, enhances the antibody responses to Env compared to non-C3d fused forms of Env, and this approach may be one way to overcome the poor ability of DNA vaccines to generate antibodies to Env.
Figures




References
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Barnett, S. W., S. Rajasekar, H. Legg, B. Doe, D. H. Fuller, J. R. Haynes, C. M. Walker, and K. S. Steimer. 1997. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine 15:869-873. - PubMed
-
- Barouch, D. H., S. Santra, M. J. Kuroda, J. E. Schmitz, R. Plishka, A. Buckler-White, A. E. Gaitan, R. Zin, J. H. Nam, L. S. Wyatt, M. A. Lifton, C. E. Nickerson, B. Moss, D. C. Montefiori, V. M. Hirsch, and N. L. Letvin. 2001. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J. Virol. 75:5151-5158. - PMC - PubMed
-
- Barouch, D. H., S. Santra, J. E. Schmitz., M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. - PubMed
-
- Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyer, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

