Duck hepatitis B virus virion secretion requires a double-stranded DNA genome
- PMID: 12525667
- PMCID: PMC140969
- DOI: 10.1128/jvi.77.3.2287-2294.2003
Duck hepatitis B virus virion secretion requires a double-stranded DNA genome
Abstract
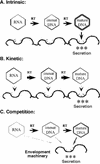
Hepatitis B virus assembly begins with the packaging of an RNA pregenome into intracellular nucleocapsids, with subsequent reverse transcription within these nucleocapsids converting the RNA into a characteristic, partially double-stranded DNA, which, alone, is found in enveloped extracellular virions as the viral genome. Using a synchronized replication system for the duck hepatitis B virus, together with a stringent two-step assay for virion secretion, we demonstrate that this selective genome secretion results from an intrinsic secretion competence gained only by the nucleocapsids at the late stage of reverse transcription.
Figures




References
-
- Jilbert, A. R., D. S. Miller, C. A. Scougall, H. Turnbull, and C. J. Burrell. 1996. Kinetics of duck hepatitis B virus infection following low dose virus inoculation: one virus DNA genome is infectious in neonatal ducks. Virology 226:338-345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

