Effect of androgen deficiency on mouse ventricular repolarization
- PMID: 12527727
- PMCID: PMC2342516
- DOI: 10.1113/jphysiol.2002.030460
Effect of androgen deficiency on mouse ventricular repolarization
Abstract
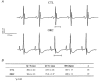
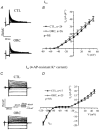
We previously demonstrated that female mouse ventricles have longer action potential durations (APDs) than males. This delayed repolarization results from a lower current density of the ultrarapid delayed rectifier K(+) current (I(K,ur)) and a lower expression level of its underlying K(+) channel (Kv1.5). To evaluate whether this sex difference could be attributable to the action of male sex hormones, we studied the effect of androgen deficiency on ventricular repolarization. We compared cardiac electrophysiological properties in castrated (orchiectomized; ORC) and control (CTL) male mice. Q-Tc intervals as well as APDs measured at 20 %, 50 % and 90 % of repolarization were all significantly longer in ORC than in CTL. The current density of I(K,ur) was significantly lower in ORC than in CTL (at +50 mV, ORC: 29 +/- 4 pA pF(-1), n = 25; CTL: 48 +/- 5 pA pF(-1), n = 17; P = 0.006). In contrast, all the other K(+) currents present in mouse ventricular myocytes were comparable between ORC and CTL. Moreover, results of Western blot analysis showed a lower expression level of Kv1.5 protein in ORC but no difference between the two groups for the other K(+) channels studied. This study demonstrates that androgen deficiency leads to a reduction in the density of I(K,ur) and Kv1.5 in mouse ventricle, and consequently, to prolongation of APD and Q-Tc interval. In conclusion, these findings strongly suggest that male sex hormones contribute to the sex difference that we previously reported in cardiac repolarization in adult mouse heart.
Figures


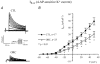


References
-
- Barry DM, Xu H, Schuessler RB, Nerbonne JM. Functional knockout of the transient outward current, long-QT syndrome, and cardiac remodelling in mice expressing a dominant-negative Kv4 α-subunit. Circ Res. 1998;83:560–567. - PubMed
-
- Bazett H. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370.
-
- Berger F, Borchard U, Hafner D, Pütz I, Weis TM. Effects of 17β-estradiol on action potential and ionic currents in male rat ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:788–796. - PubMed
-
- Bidoggia H, Maciel JP, Capalozza N, Mosca S, Blaksley EJ, Valverde E, Bertran G, Arini P, Biagetti MO, Quinteiro R. Sex difference on the electrocardiographic pattern of cardiac repolarization: possible role of testosterone. Am Heart J. 2000;140:678–683. - PubMed
-
- Chou TM, Sudhir K, Hutchison SJ, Ko E, Amidon TM, Collins P, Chatterjee K. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation. 1996;94:2614–2619. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

