Parallel decrease in omega-conotoxin-sensitive transmission and dopamine-induced inhibition at the striatal synapse of developing rats
- PMID: 12527734
- PMCID: PMC2342531
- DOI: 10.1113/jphysiol.2002.031773
Parallel decrease in omega-conotoxin-sensitive transmission and dopamine-induced inhibition at the striatal synapse of developing rats
Abstract
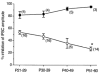
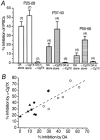
Whole-cell patch-clamp recordings of GABAergic IPSCs were made from cholinergic interneurones in slices of striatum from developing rats aged 21-60 days postnatal. In addition, the Ca(2+) channel subtypes involved in synaptic transmission, as well as dopamine (DA)-induced presynaptic inhibition, were investigated pharmacologically with development by bath application of Ca(2+) channel blockers and DA receptor agonists. The IPSC amplitude was reduced by omega-conotoxin GVIA (omega-CgTX) or omega-agatoxin TK (omega-Aga-TK) across the whole age range, suggesting that multiple types of Ca(2+) channels mediate transmission of the synapse. The IPSC fraction reduced by omega-CgTX significantly decreased, whereas that reduced by omega-Aga-TK remained unchanged with development. DA or quinpirole, a D(2)-like receptor agonist, presynaptically reduced the IPSC amplitude throughout development. The DA-induced inhibition decreased with age in parallel with the decrease in N-type Ca(2+) channels. DA showed no further inhibition of IPSCs after the inhibitory effect of omega-CgTX had reached steady state throughout development. These results demonstrate that there is a functional link between presynaptic N-type Ca(2+) channels and D(2)-like DA receptors at inhibitory synapses in the striatum. They also demonstrate that the suppression of GABAergic transmission by D(2)-like receptors is mediated by modulation of N-type Ca(2+) channels and decreases in parallel with the developmental decline in the contribution of N-type Ca(2+) channels to exocytosis.
Figures




References
-
- Barnes-Davies M, Owens S, Forsythe ID. Calcium channels triggering transmitter release in the rat medial superior olive. Hear Res. 2001;162:134–145. - PubMed
-
- Bolam P, Wainer BH, Smith AD. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neurosci. 1984;12:711–718. - PubMed
-
- Broaddus WC, Bennett JP., Jr Postnatal development of striatal dopamine function. I. An examination of D1 and D2 receptors, adenylate cyclase regulation and presynaptic dopamine markers. Brain Res Mol Brain Res. 1990;52:265–271. - PubMed
-
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23:120–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

