Uptake of locally applied deoxyglucose, glucose and lactate by axons and Schwann cells of rat vagus nerve
- PMID: 12527741
- PMCID: PMC2342518
- DOI: 10.1113/jphysiol.2002.029751
Uptake of locally applied deoxyglucose, glucose and lactate by axons and Schwann cells of rat vagus nerve
Abstract
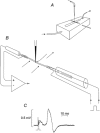
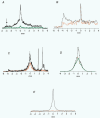
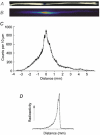
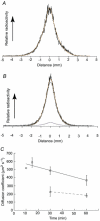
We asked whether, in a steady state, neurons and glial cells both take up glucose sufficient for their energy requirements, or whether glial cells take up a disproportionate amount and transfer metabolic substrate to neurons. A desheathed rat vagus nerve was held crossways in a laminar flow perfusion chamber and stimulated at 2 Hz. (14)C-labelled substrate was applied from a micropipette for 5 min over a < 0.6 mm band of the surface of the nerve. After 10-55 min incubation, the nerve was lyophilized and the longitudinal distribution of radioactivity measured. When the weakly metabolizable analogue of glucose, 2-deoxy-[U-(14)C]D-glucose (*DG), was applied, the profiles of the radioactivity broadened with time, reaching distances several times the mean length of the Schwann cells (0.32 mm; most of the Schwann cells are non-myelinating). The profiles were well fitted by curves calculated for diffusion in a single compartment, the mean diffusion coefficient being 463 +/- 34 microm(2) s(-1) (+/- S.E.M., n = 16). Applications of *DG were repeated in the presence of the gap junction blocker, carbenoxolone (100 microM). The profiles were now narrower and better fitted with two compartments. One compartment had a coefficient not significantly different from that in the absence of the gap junction blocker (axons), the other compartment had a coefficient of 204 +/- 24 microm(2) s(-1), n = 4. Addition of the gap junction blocker 18-alpha-glycyrrhetinic acid, or blocking electrical activity with TTX, also reduced longitudinal diffusion. Ascribing the compartment in which diffusion was reduced by these treatments to non-myelinating Schwann cells, we conclude that 78.0 +/- 3.6 % (n = 9) of the uptake of *DG was into Schwann cells. This suggests that there was transfer of metabolic substrate from Schwann cells to axons. Local application of [(14)C]glucose or [(14)C]lactate led to variable labelling along the length of the nerve, but with both substrates narrow peaks were often present at the application site; these were greatly reduced by subsequent treatment with amylase, a glycogen-degrading enzyme.
Figures

Comment in
-
How to balance the brain energy budget while spending glucose differently.J Physiol. 2003 Jan 15;546(Pt 2):325. doi: 10.1113/jphysiol.2002.035105. J Physiol. 2003. PMID: 12527720 Free PMC article. No abstract available.
References
-
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. - PubMed
-
- Bachelard HS. Specificity and kinetic properties of monosaccharide uptake into guinea pig cerebral cortex in vitro. J Neurochem. 1971;18:213–222. - PubMed
-
- Bouzier AK, Thiaudiere E, Biran M, Rouland R, Canioni P, Merle M. The metabolism of [3-13C]lactate in the rat brain is specific of a pyruvate carboxylase-deprived compartment. J Neurochem. 2000;75:480–486. - PubMed
-
- Brooks GA. Lactate shuttles in nature. Biochem Soc Trans. 2002;30:258–264. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

