Spatio-temporal activation of caspase revealed by indicator that is insensitive to environmental effects
- PMID: 12527749
- PMCID: PMC2172647
- DOI: 10.1083/jcb.200207111
Spatio-temporal activation of caspase revealed by indicator that is insensitive to environmental effects
Abstract
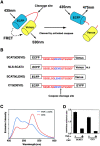
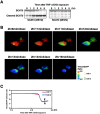
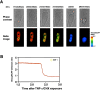
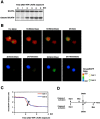
Indicator molecules for caspase-3 activation have been reported that use fluorescence resonance energy transfer (FRET) between an enhanced cyan fluorescent protein (the donor) and enhanced yellow fluorescent protein (EYFP; the acceptor). Because EYFP is highly sensitive to proton (H+) and chloride ion (Cl-) levels, which can change during apoptosis, this indicator's ability to trace the precise dynamics of caspase activation is limited, especially in vivo. Here, we generated an H+- and Cl--insensitive indicator for caspase activation, SCAT, in which EYFP was replaced with Venus, and monitored the spatio-temporal activation of caspases in living cells. Caspase-3 activation was initiated first in the cytosol and then in the nucleus, and rapidly reached maximum activation in 10 min or less. Furthermore, the nuclear activation of caspase-3 preceded the nuclear apoptotic morphological changes. In contrast, the completion of caspase-9 activation took much longer and its activation was attenuated in the nucleus. However, the time between the initiation of caspase-9 activation and the morphological changes was quite similar to that seen for caspase-3, indicating the activation of both caspases occurred essentially simultaneously during the initiation of apoptosis.
Figures



References
-
- Araki, T., M. Hayashi, N. Watanabe, H. Kanuka, J. Yoshino, M. Miura, and T. Saruta. 2002. Down-regulation of Mcl-1 by inhibition of the PI3-K/Akt pathway is required for cell shrinkage-dependent cell death. Biochem. Biophys. Res. Commun. 290:1275–1281. - PubMed
-
- Cecconi, F., G. Alvarez-Bolado, B.I. Meyer, K.A. Roth, and P. Gruss. 1998. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 94:727–737. - PubMed
-
- Chandler, J.M., G.M. Cohen, and M. MacFarlane. 1998. Different subcellular distribution of caspase-3 and caspase-7 following Fas-induced apoptosis in mouse liver. J. Biol. Chem. 273:10815–10818. - PubMed
-
- Cryns, V., and J. Yuan. 1998. Proteases to die for. Genes Dev. 12:1551–1570. - PubMed
-
- Eguchi, Y., A. Srinivasan, K.J. Tomaselli, S. Shimizu, and Y. Tsujimoto. 1999. ATP-dependent steps in apoptotic signal transduction. Cancer Res. 59:2174–2181. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

