Binding of an ankyrin-1 isoform to obscurin suggests a molecular link between the sarcoplasmic reticulum and myofibrils in striated muscles
- PMID: 12527750
- PMCID: PMC2172649
- DOI: 10.1083/jcb.200208109
Binding of an ankyrin-1 isoform to obscurin suggests a molecular link between the sarcoplasmic reticulum and myofibrils in striated muscles
Abstract
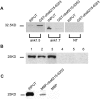
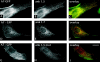
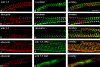
Assembly of specialized membrane domains, both of the plasma membrane and of the ER, is necessary for the physiological activity of striated muscle cells. The mechanisms that mediate the structural organization of the sarcoplasmic reticulum with respect to the myofibrils are, however, not known. We report here that ank1.5, a small splice variant of the ank1 gene localized on the sarcoplasmic reticulum membrane, is capable of interacting with a sequence of 25 aa located at the COOH terminus of obscurin. Obscurin is a giant sarcomeric protein of approximately 800 kD that binds to titin and has been proposed to mediate interactions between myofibrils and other cellular structures. The binding sites and the critical aa required in the interaction between ank1.5 and obscurin were characterized using the yeast two-hybrid system, in in vitro pull-down assays and in experiments in heterologous cells. In differentiated skeletal muscle cells, a transfected myc-tagged ank1.5 was found to be selectively restricted near the M line region where it colocalized with endogenous obscurin. The M line localization of ank1.5 required a functional obscurin-binding site, because mutations of this domain resulted in a diffused distribution of the mutant ank1.5 protein in skeletal muscle cells. The interaction between ank1.5 and obscurin represents the first direct evidence of two proteins that may provide a direct link between the sarcoplasmic reticulum and myofibrils. In keeping with the proposed role of obscurin in mediating an interaction with ankyrins and sarcoplasmic reticulum, we have also found that a sequence with homology to the obscurin-binding site of ank1.5 is present in the ank2.2 isoform, which in striated muscles has been also shown to associate with the sarcoplasmic reticulum. Accordingly, a peptide containing the COOH terminus of ank2.2 fused with GST was found to bind to obscurin. Based on reported evidence showing that the COOH terminus of ank2.2 is necessary for the localization of ryanodine receptors and InsP3 receptors in the sarcoplasmic reticulum, we propose that obscurin, through multiple interactions with ank1.5 and ank2.2 isoforms, may assemble a large protein complex that, in addition to a structural function, may play a role in the organization of specific subdomains in the sarcoplasmic reticulum.
Figures


References
-
- Bang, M.L., T. Centner, F. Fornoff, A.J. Geach, M. Gotthardt, M. McNabb, C.C. Witt, D. Labeit, C.C. Gregorio, H. Granzier, and S. Labeit. 2001. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ. Res. 89:1065–1072. - PubMed
-
- Baumann, O., and B. Walz. 2001. Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int. Rev. Cytol. 205:149–214. - PubMed
-
- Beck, K.A., J.A. Buchanan, and W.J. Nelson. 1997. Golgi membrane skeleton: identification, localization and oligomerization of a 195 kDa ankyrin isoform associated with the Golgi complex. J. Cell Sci. 110:1239–1249. - PubMed
-
- Bellin, R.B., T.W. Huiatt, D.R. Critchley, and R.M. Robson. 2001. Synemin may function to directly link muscle cell intermediate filaments to both myofibrillar Z-lines and costameres. J. Biol. Chem. 276:32330–32337. - PubMed
-
- Bennett, V., and A.J. Baines. 2001. Spectrin and ankyrin-based pathways: metazoan invenctions for integrating cells into tissues. Physiol. Rev. 81:1353–1392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

