Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis
- PMID: 12527751
- PMCID: PMC2172637
- DOI: 10.1083/jcb.200209006
Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis
Abstract
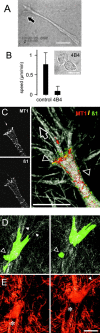
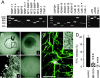
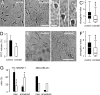
Invasive tumor dissemination in vitro and in vivo involves the proteolytic degradation of ECM barriers. This process, however, is only incompletely attenuated by protease inhibitor-based treatment, suggesting the existence of migratory compensation strategies. In three-dimensional collagen matrices, spindle-shaped proteolytically potent HT-1080 fibrosarcoma and MDA-MB-231 carcinoma cells exhibited a constitutive mesenchymal-type movement including the coclustering of beta 1 integrins and MT1-matrix metalloproteinase (MMP) at fiber bindings sites and the generation of tube-like proteolytic degradation tracks. Near-total inhibition of MMPs, serine proteases, cathepsins, and other proteases, however, induced a conversion toward spherical morphology at near undiminished migration rates. Sustained protease-independent migration resulted from a flexible amoeba-like shape change, i.e., propulsive squeezing through preexisting matrix gaps and formation of constriction rings in the absence of matrix degradation, concomitant loss of clustered beta 1 integrins and MT1-MMP from fiber binding sites, and a diffuse cortical distribution of the actin cytoskeleton. Acquisition of protease-independent amoeboid dissemination was confirmed for HT-1080 cells injected into the mouse dermis monitored by intravital multiphoton microscopy. In conclusion, the transition from proteolytic mesenchymal toward nonproteolytic amoeboid movement highlights a supramolecular plasticity mechanism in cell migration and further represents a putative escape mechanism in tumor cell dissemination after abrogation of pericellular proteolysis.
Figures




References
-
- Aimes, R.T., and J.P. Quigley. 1995. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J. Biol. Chem. 270:5872–5876. - PubMed
-
- Bachmeier, B.E., A.G. Nerlich, R. Lichtinghagen, and C.P. Sommerhoff. 2001. Matrix metalloproteinases (MMPs) in breast cancer cell lines of different tumorigenicity. Anticancer Res. 21:3821–3828. - PubMed
-
- Bedi, G.S., and T. Williams. 1994. Purification and characterization of a collagen-degrading protease from Porphyromonas gingivalis. J. Biol. Chem. 269:599–606. - PubMed
-
- Belkin, A.M., S.S. Akimov, L.S. Zaritskaya, B.I. Ratnikov, E.I. Deryugina, and A.Y. Strongin. 2001. Matrix-dependent proteolysis of surface transglutaminase by membrane-type metalloproteinase regulates cancer cell adhesion and locomotion. J. Biol. Chem. 276:18415–18422. - PubMed
-
- Birchmeier, W., and C. Birchmeier. 1995. Epithelial-mesenchymal transitions in development and tumor progression. EXS. 74:1–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

