Fluoride-cleavable biotinylation phosphoramidite for 5'-end-labeling and affinity purification of synthetic oligonucleotides
- PMID: 12527780
- PMCID: PMC140496
- DOI: 10.1093/nar/gkg130
Fluoride-cleavable biotinylation phosphoramidite for 5'-end-labeling and affinity purification of synthetic oligonucleotides
Abstract
A fluoride-cleavable phosphoramidite for biotinylation was designed, synthesized and coupled efficiently to the 5'-end of DNA on an automatic synthesizer. The diisopropylsilyl acetal functionality was used to link the biotin moiety through a tertiary hydroxide group to the 5'-end of DNA. This linkage proved to be completely stable under certain post-synthetic DNA cleavage/deprotection conditions [0.05 M K(2)CO(3) in MeOH, room temperature, 24 h and MeNH(2) (approximately 40%)/NH(4)OH (approximately 29%), 1:1 v/v, 65 degrees C, 30 min] while it can be readily broken by fluoride ion, releasing unmodified DNA. To demonstrate the use of this DNA biotinylation method, we applied this method in affinity purification of synthetic DNA. As revealed by HPLC analysis, biotinylated full-length DNA can be efficiently attached to NeutrAvidin coated microspheres, and failure sequences can be readily removed. Subsequent treatment of the microspheres with pyridine/HF released high quality full-length unmodified DNA in good yield.
Figures



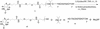
References
-
- McInnes J.L. and Symons,R.H. (1989) Preparation and detection of nonradioactive nucleic acid and oligonucleotide probes. In Symons,R.H. (ed.), Nucleic Acid Probes. CRC Press, Boca Raton, FL, pp. 33–80.
-
- Gildea B.D., Coull,J.M. and Koster,H. (1990) A versatile acid-labile linker for modification of synthetic biomolecules. Tetrahedron Lett., 31, 7095–7098.
-
- De Vos M.J., Van Elsen,A. and Bollen,A. (1994) New non-nucleosidic phosphoramidites for the solid phase multi-labeling of oligonucleotides: comb- and multifork-like structure. Nucleosides Nucleotides, 13, 2245–2265.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

