Proteasome inhibition results in TRAIL sensitization of primary keratinocytes by removing the resistance-mediating block of effector caspase maturation
- PMID: 12529384
- PMCID: PMC140698
- DOI: 10.1128/MCB.23.3.777-790.2003
Proteasome inhibition results in TRAIL sensitization of primary keratinocytes by removing the resistance-mediating block of effector caspase maturation
Abstract
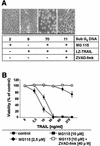
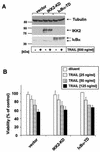
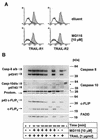
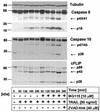
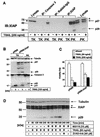
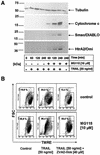
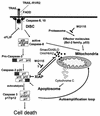
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) exerts potent cytotoxic activity against transformed keratinocytes, whereas primary keratinocytes are relatively resistant. In several cell types, inhibition of the proteasome sensitizes for TRAIL-induced apoptosis by interference with NF-kappaB activation. Here we describe a novel intracellular mechanism of TRAIL resistance in primary cells and how this resistance is removed by proteasome inhibitors independent of NF-kappaB in primary human keratinocytes. This sensitization was not mediated at the receptor-proximal level of TRAIL DISC formation or caspase 8 activation but further downstream. Activation of caspase 3 was critical, as it only occurred when mitochondrial apoptotic pathways were activated, as reflected by Smac/DIABLO, HtrA2, and cytochrome c release. Smac/DIABLO and HtrA2 are needed to release the X-linked inhibitor-of-apoptosis protein (XIAP)-mediated block of full caspase 3 maturation. XIAP can effectively block caspase 3 maturation and, intriguingly, is highly expressed in primary but not in transformed keratinocytes. Ectopic XIAP expression in transformed keratinocytes resulted in increased resistance to TRAIL. Our data suggest that breaking of this resistance via proteasome inhibitors, which are potential anticancer drugs, may sensitize certain primary cells to TRAIL-induced apoptosis and could thereby complicate the clinical applicability of a combination of TRAIL receptor agonists with proteasome inhibitors.
Figures



Similar articles
-
X-linked inhibitor of apoptosis (XIAP) blocks Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis of prostate cancer cells in the presence of mitochondrial activation: sensitization by overexpression of second mitochondria-derived activator of caspase/direct IAP-binding protein with low pl (Smac/DIABLO).Mol Cancer Ther. 2002 Oct;1(12):1051-8. Mol Cancer Ther. 2002. PMID: 12481428
-
Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis of human melanoma is regulated by smac/DIABLO release from mitochondria.Cancer Res. 2001 Oct 1;61(19):7339-48. Cancer Res. 2001. PMID: 11585775
-
Influence of casein kinase II in tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human rhabdomyosarcoma cells.Clin Cancer Res. 2004 Oct 1;10(19):6650-60. doi: 10.1158/1078-0432.CCR-04-0576. Clin Cancer Res. 2004. PMID: 15475455
-
Regulation of TRAIL-induced apoptosis by ectopic expression of antiapoptotic factors.Vitam Horm. 2004;67:453-83. doi: 10.1016/S0083-6729(04)67023-3. Vitam Horm. 2004. PMID: 15110190 Review.
-
Proteasomes in apoptosis: villains or guardians?Cell Mol Life Sci. 1999 Dec;56(11-12):908-17. doi: 10.1007/s000180050483. Cell Mol Life Sci. 1999. PMID: 11212325 Free PMC article. Review.
Cited by
-
Novel insights into the synergistic interaction of Bortezomib and TRAIL: tBid provides the link.Oncotarget. 2011 May;2(5):418-21. doi: 10.18632/oncotarget.277. Oncotarget. 2011. PMID: 21789791 Free PMC article.
-
Nitric oxide-mediated sensitization of resistant tumor cells to apoptosis by chemo-immunotherapeutics.Redox Biol. 2015 Dec;6:486-494. doi: 10.1016/j.redox.2015.08.013. Epub 2015 Aug 18. Redox Biol. 2015. PMID: 26432660 Free PMC article. Review.
-
Treating metastatic solid tumors with bortezomib and a tumor necrosis factor-related apoptosis-inducing ligand receptor agonist antibody.J Natl Cancer Inst. 2008 May 7;100(9):649-62. doi: 10.1093/jnci/djn113. Epub 2008 Apr 29. J Natl Cancer Inst. 2008. PMID: 18445820 Free PMC article.
-
The promise of TRAIL--potential and risks of a novel anticancer therapy.J Mol Med (Berl). 2007 Sep;85(9):923-35. doi: 10.1007/s00109-007-0194-1. Epub 2007 Apr 17. J Mol Med (Berl). 2007. PMID: 17437073 Review.
-
Upstream regulatory role for XIAP in receptor-mediated apoptosis.Mol Cell Biol. 2004 Aug;24(16):7003-14. doi: 10.1128/MCB.24.16.7003-7014.2004. Mol Cell Biol. 2004. PMID: 15282301 Free PMC article.
References
-
- Ashkenazi, A., R. C. Pai, S. Fong, S. Leung, D. A. Lawrence, S. A. Marsters, C. Blackie, L. Chang, A. E. McMurtrey, A. Hebert, L. DeForge, I. L. Koumenis, D. Lewis, L. Harris, J. Bussiere, H. Koeppen, Z. Shahrokh, and R. H. Schwall. 1999. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Investig. 104:155-162. - PMC - PubMed
-
- Beg, A. A., W. C. Sha, R. T. Bronson, S. Ghosh, and D. Baltimore. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376:167-170. - PubMed
-
- Bodmer, J. L., N. Holler, S. Reynard, P. Vinciguerra, P. Schneider, P. Juo, J. Blenis, and J. Tschopp. 2000. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat. Cell Biol. 2:241-243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
