Recruitment of a repressosome complex at the growth hormone receptor promoter and its potential role in diabetic nephropathy
- PMID: 12529387
- PMCID: PMC140700
- DOI: 10.1128/MCB.23.3.815-825.2003
Recruitment of a repressosome complex at the growth hormone receptor promoter and its potential role in diabetic nephropathy
Abstract
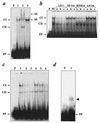
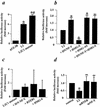
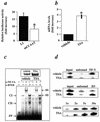
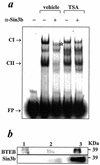
The growth hormone (GH)-GH receptor (GHR) axis modulates growth and metabolism and contributes to complications of diabetes mellitus. We analyzed the promoter region of the dominant transcript (L2) of the murine GHR to determine that a cis element, L2C1, interacts with transcription factors NF-Y, BTEB1, and HMG-Y/I. These proteins individually repress GHR expression and together form a repressosome complex in conjunction with mSin3b. The histone deacetylase inhibitor trichostatin A increases expression of the murine GHR gene, enhances association of acetyl-H3 at L2C1, inhibits formation of the repressosome complex, and decreases NF-Y's association with L2C1. Our studies reveal that murine models of experimental diabetes mellitus are characterized by reduced hepatic GHR expression, decreased acetyl-H3 associated with L2C1, and increased formation of the repressosome complex. In contrast, in the kidney diabetes mellitus is associated with enhanced GHR expression and lack of alteration in the assembly of the repressosome complex, thus permitting exposure of kidneys to the effects of elevated levels of GH in diabetes mellitus. Our findings define a higher-order repressosome complex whose formation correlates with the acetylation status of chromatin histone proteins. The delineation of the role of this repressosome complex in regulating tissue-specific expression of GHR in diabetes mellitus provides a molecular model for the role of GH in the genesis of certain microvascular complications of diabetes mellitus.
Figures



References
-
- Baxter, R. C., J. M. Bryson, and J. R. Turtle. 1980. Somatogenic receptors of rat liver: regulation by insulin. Endocrinology 107:1176-1181. - PubMed
-
- Bellush, L. L., S. Doublier, A. N. Holland, L. J. Striker, G. E. Striker, and J. J. Kopchick. 2000. Protection against diabetes-induced nephropathy in growth hormone receptor/binding protein gene-disrupted mice. Endocrinology 141:163-168. - PubMed
-
- Cheung, P., C. D. Allis, and P. Sassone-Corsi. 2000. Signaling to chromatin through histone modifications. Cell 103:263-271. - PubMed
-
- Currie, R. A. 1997. Functional interaction between the DNA binding subunit trimerization domain of NF-Y and the high mobility group protein HMG-I(Y). J. Biol. Chem. 272:30880-30888. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
