Homeodomain-interacting protein kinase 1 modulates Daxx localization, phosphorylation, and transcriptional activity
- PMID: 12529400
- PMCID: PMC140690
- DOI: 10.1128/MCB.23.3.950-960.2003
Homeodomain-interacting protein kinase 1 modulates Daxx localization, phosphorylation, and transcriptional activity
Abstract
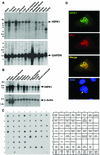
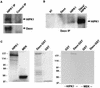
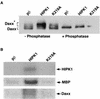
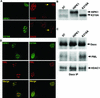
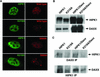
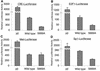
We describe an interaction between homeodomain-interacting protein kinase 1 (HIPK1) and Daxx, two transcriptional regulators important in transducing growth-regulatory signals. We demonstrate that HIPK1 is ubiquitously expressed in mice and humans and localizes predominantly to the nucleus. Daxx normally resides within the nucleus in promyelocytic leukemia protein (PML) oncogenic domains (PODs), where it physically interacts with PML. Under certain circumstances, Daxx is relocalized from PODs to chromatin, where it then acts as a transcriptional repressor through an association with histone deacetylase (HDAC1). We propose two novel mechanisms for regulating the activity of Daxx, both mediated by HIPK1. First, HIPK1 physically interacts with Daxx in cells and consequently relocalizes Daxx from PODs. Daxx relocalization disrupts its interaction with PML and augments its interaction with HDAC1, likely influencing Daxx activity. Although the relocalization of Daxx from PODs is phosphorylation independent, an active HIPK1 kinase domain is required, suggesting that HIPK1 autophosphorylation is important in this interaction. Second, HIPK1 phosphorylates Daxx on Ser 669, and phosphorylation of this site is important in modulating the ability of Daxx to function as a transcriptional repressor. Mutation of Daxx Ser 669 to Ala results in increased repression in three of four transcriptional reporters, suggesting that phosphorylation by HIPK1 diminishes Daxx transcriptional repression of specific promoters. Taken together, our results indicate that HIPK1 and Daxx collaborate in regulating transcription.
Figures

Similar articles
-
Role of the ASK1-SEK1-JNK1-HIPK1 signal in Daxx trafficking and ASK1 oligomerization.J Biol Chem. 2003 Nov 21;278(47):47245-52. doi: 10.1074/jbc.M213201200. Epub 2003 Sep 10. J Biol Chem. 2003. PMID: 12968034
-
Identification of Daxx interacting with p73, one of the p53 family, and its regulation of p53 activity by competitive interaction with PML.Nucleic Acids Res. 2003 Sep 15;31(18):5356-67. doi: 10.1093/nar/gkg741. Nucleic Acids Res. 2003. PMID: 12954772 Free PMC article.
-
Sequestration and inhibition of Daxx-mediated transcriptional repression by PML.Mol Cell Biol. 2000 Mar;20(5):1784-96. doi: 10.1128/MCB.20.5.1784-1796.2000. Mol Cell Biol. 2000. PMID: 10669754 Free PMC article.
-
The Dynamic Regulation of Daxx-Mediated Transcriptional Inhibition by SUMO and PML NBs.Int J Mol Sci. 2025 Jul 12;26(14):6703. doi: 10.3390/ijms26146703. Int J Mol Sci. 2025. PMID: 40724953 Free PMC article. Review.
-
PML NBs (ND10) and Daxx: from nuclear structure to protein function.Front Biosci. 2008 May 1;13:7132-42. doi: 10.2741/3216. Front Biosci. 2008. PMID: 18508722 Review.
Cited by
-
Effect of tyrosine autophosphorylation on catalytic activity and subcellular localisation of homeodomain-interacting protein kinases (HIPK).Cell Commun Signal. 2015 Jan 29;13:3. doi: 10.1186/s12964-014-0082-6. Cell Commun Signal. 2015. PMID: 25630557 Free PMC article.
-
HIPK1 drives p53 activation to limit colorectal cancer cell growth.Cell Cycle. 2013 Jun 15;12(12):1879-91. doi: 10.4161/cc.24927. Epub 2013 May 15. Cell Cycle. 2013. PMID: 23676219 Free PMC article.
-
Death-associated protein 6 (Daxx) mediates cAMP-dependent stimulation of Cyp11a1 (P450scc) transcription.J Biol Chem. 2012 Feb 17;287(8):5910-6. doi: 10.1074/jbc.M111.307603. Epub 2011 Dec 24. J Biol Chem. 2012. PMID: 22199361 Free PMC article.
-
Cyclic AMP stimulates SF-1-dependent CYP11A1 expression through homeodomain-interacting protein kinase 3-mediated Jun N-terminal kinase and c-Jun phosphorylation.Mol Cell Biol. 2007 Mar;27(6):2027-36. doi: 10.1128/MCB.02253-06. Epub 2007 Jan 8. Mol Cell Biol. 2007. PMID: 17210646 Free PMC article.
-
A test for ancient selective sweeps and an application to candidate sites in modern humans.Mol Biol Evol. 2014 Dec;31(12):3344-58. doi: 10.1093/molbev/msu255. Epub 2014 Aug 28. Mol Biol Evol. 2014. PMID: 25172957 Free PMC article.
References
-
- Amin, H. M., S. Saeed, and S. Alkan. 2001. Histone deacetylase inhibitors induce caspase-dependent apoptosis and downregulation of daxx in acute promyelocytic leukaemia with t(15;17). Br. J. Haematol. 115:287-297. - PubMed
-
- Cermak, L., A. Imova, A. Pintzas, V. Horeji, and L. Andera. 2001. Molecular mechanisms involved in CD43-mediated apoptosis of TF-1 cells: roles of transcription, Daxx expression and adhesion molecules. J. Biol. Chem. 31:31. - PubMed
-
- Chang, H. Y., H. Nishitoh, X. Yang, H. Ichijo, and D. Baltimore. 1998. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 281:1860-1863. - PubMed
-
- Choi, C. Y., Y. H. Kim, H. J. Kwon, and Y. Kim. 1999. The homeodomain protein NK-3 recruits Groucho and a histone deacetylase complex to repress transcription. J. Biol. Chem. 274:33194-33197. - PubMed
-
- D'Orazi, G., B. Cecchinelli, T. Bruno, I. Manni, Y. Higashimoto, S. Saito, M. Gostissa, S. Coen, A. Marchetti, G. Del Sal, G. Piaggio, M. Fanciulli, E. Appella, and S. Soddu. 2002. Homeodomain-interacting protein kinase-2 phosphorylates p53 at Ser 46 and mediates apoptosis. Nat. Cell Biol. 4:11-19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
